Translate this page into:
Spontaneous Retroperitoneal Hemorrhage - A Rare Presentation of Polyarteritis Nodosa: Role of Angiography in Diagnosis and Management

Corresponding Author: Pranav Sharma, Department of Radiology, Yale New Haven Health Bridgeport Hospital, 267 Grant Street, Bridgeport, Connecticut - 06610, United States. E-mail: drpranavsharma29@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Sharma P, Kochar P, Sharma S, Rotem E. Spontaneous Retroperitoneal Hemorrhage - A Rare Presentation of Polyarteritis Nodosa: Role of Angiography in Diagnosis and Management. Am J Interv Radiol 2019;3(2):1-5.
Abstract
Spontaneous retroperitoneal hemorrhage (RPH) is a rare but serious complication of polyarteritis nodosa (PAN) and must be considered in patients presenting with RPH as their first presentation. Renal infarctions, liver infarctions, and ruptured microaneurysms are all complications of PAN. We present two cases presenting with abdominal and back pain. The first patient’s abdominal computed tomography (CT) scan revealed fractured right kidney with retroperitoneal pericapsular hematoma and multiple hepatic and splenic infarcts. The digital subtraction angiography (DSA) demonstrated large areas of devascularization of the right kidney and right renal arterial wall irregularity with multifocal areas of stenosis, dilatations, and microaneurysms without active extravasation of IV contrast. She was treated conservatively and started on a pulsed dose of steroids and cyclophosphamide. The second patient’s abdominal CT angiography revealed multiple visceral aneurysms and focal areas of stenosis in branches of celiac axis and superior mesentery artery without active contrast extravasation. The DSA demonstrated multifocal areas of irregularity and narrowing in celiac and intrahepatic arteries as well as a 9 mm pseudoaneurysm in the inferior pancreaticoduodenal artery which was embolized with metallic coils. PAN has a vague clinical presentation and is clinically occult. Patients may be diagnosed while getting investigated for some other causes of abdominal pain. The emergency physician and the radiologist should be aware of the findings and should be able to correlate with pathology to prevent life-threatening complications. Angiography plays a crucial role, not only in diagnosis but also in appropriate management.
Keywords
Embolization
Microaneurysms
Polyarteritis nodosa
Retroperitoneal hemorrhage
Spontaneous renal hemorrhage
INTRODUCTION
Polyarteritis nodosa (PAN) is necrotizing vasculitis involving medium- or small-sized arteries and leads to microaneurysms formation. Most patients present with vague symptoms and may rarely present with life-threatening complications such as retroperitoneal hemorrhage (RPH). Institutional Review Board exemption was obtained regarding the study due to its retrospective nature and low sample size.
CASE REPORT
Case 1
A 46-year-old female, initially, presented with right upper quadrant pain associated with nausea without a history of diarrhea, fever, or dysuria. Physical examination revealed right upper quadrant tenderness with positive Murphy sign, bilateral costovertebral angle tenderness, and epigastric tenderness. Computed tomography (CT) scan revealed right renal infarct. Magnetic resonance angiography of abdomen performed a few days later revealed a new left renal infarct. The differential diagnosis of atrial fibrillation, atherosclerotic embolism, hypercoagulable state, and fibromuscular dysplasia were considered. Her antineutrophil cytoplasmic antibody (ANCA) was negative. Transesophageal echocardiography, Doppler of lower extremities, and other laboratory hypercoagulable state workup were negative; however, the patient was started on anticoagulation. Subsequently, 2 weeks later, she presented with right lower quadrant (RLQ) abdominal pain, diaphoresis, and hypotension. CT scan revealed fractured right kidney with retroperitoneal pericapsular hematoma and multiple hepatic and splenic infarcts (Figure 1). Interventional radiology was consulted for possible embolization of bleeding vessel. The digital subtraction angiography (DSA) performed by experienced interventional radiologist demonstrated fractured right kidney with a large area of devascularization. Multiple areas of vessel wall irregularity with multifocal areas of stenosis, dilatations, and microaneurysms were also seen in the right renal artery (Figure 2). Mild beading of the origin of the left renal artery was also noted. No active extravasation of IV contrast was demonstrated. She was treated conservatively and started on pulsed steroids and cyclophosphamide. On the 6 months follow-up, the patient was stable and was on medical management with methotrexate and folic acid.
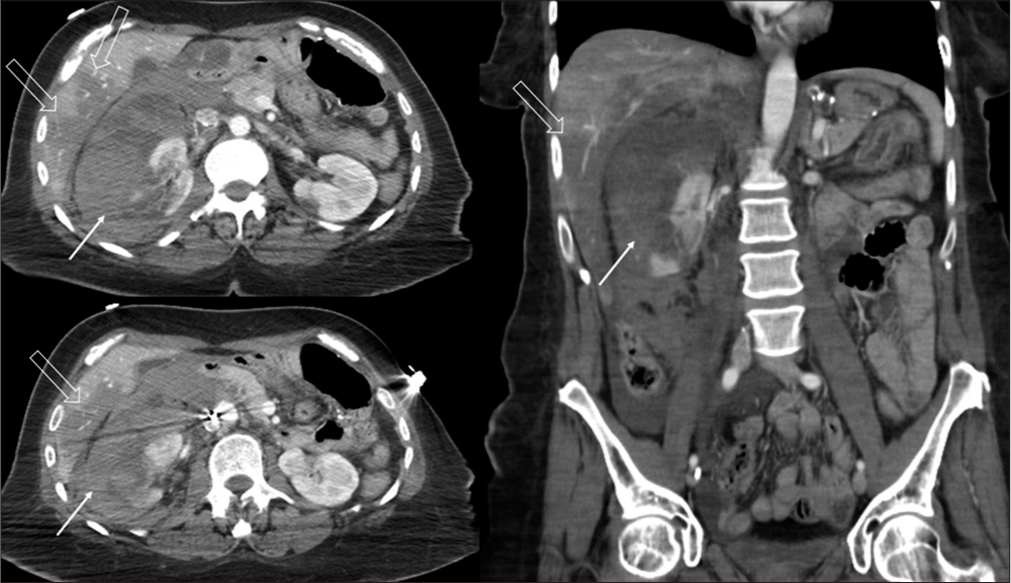
- A 46-year-old female with abdominal pain. The axial and coronal computed tomography images of abdomen demonstrate fractured right kidney with a large perinephric retroperitoneal hematoma (white solid arrow) and also noted are multiple wedge-shaped hypodensities in liver consistent with infarcts (white hollow arrow).

- A 46-year-old female with abdominal pain. Right renal angiogram demonstrates severe irregularity with multifocal areas of vascular stenosis (white hollow arrow), dilatations, and microaneurysms (white solid arrow) in the right arteries with large areas of devascularization shown by only the upper pole of kidney is opacified with contrast seen as a faint blush of contrast (Curved arrows). The lower pole and the lateral aspect of the upper pole of the right kidney are not visualized, likely devascularized. Incidental note is made of Greenfield Inferior vena cava filter.
Case 2
A 61-year-old female with hypertension and chronic lower quadrant pain presents to the emergency department with RLQ abdominal pain, nausea, vomiting, and anterior chest pain for 3 days. She had a history of left common iliac vein stent for May-Thurner syndrome (Iliofemoral deep venous thrombosis secondary to compression of the left common iliac vein by the right common iliac artery). CT scan of the abdomen revealed hemorrhage in the right anterior pararenal region. She was hemodynamically stable, but there was a hemoglobin drop from 14.2 mm of Hg to 12.2 mm of Hg. Serum lactate at the time of presentation was 5.2 which later dropped to 2.7 with resuscitation. Her ANCA was negative. CT angiography (CTA), performed subsequently, revealed multiple visceral aneurysms and focal areas of stenosis in the branches of the celiac artery and superior mesenteric artery without active contrast extravasation. Notably, there was a 9 mm pseudoaneurysm in the inferior pancreaticoduodenal artery in the center of the retroperitoneal hematoma (Figure 3). The DSA performed by experienced interventional radiologist revealed multifocal areas of irregularity and strictures in the pancreaticoduodenal branches of the superior mesenteric artery and inferior pancreaticoduodenal pseudoaneurysm (Figure 4). The opacified celiac artery and its branches were irregular with multifocal areas of luminal stenosis and post-stenotic dilatation. Embolization of pseudoaneurysm was then carried out using metallic coils using a sandwich technique (Figure 5). Post-embolization there was no flow through the inferior pancreaticoduodenal artery with good flow through the collaterals to the pancreaticoduodenal bed.

- A 61-year-old female with abdominal pain. The axial and coronal computed tomography images of abdomen demonstrating large right anterior perirenal space retroperitoneal hematoma with inferior pancreaticoduodenal pseudoaneurysm (white solid arrow) within the hematoma without active extravasation of IV contrast. Volume rendered three-dimensional image showing the inferior pancreaticoduodenal pseudoaneurysm (white solid arrow) with proximal vascular irregularity and also note multiple areas of luminal dilatation and stenosis (White hollow arrow) involving multiple other arteries such as left hepatic artery, gastroduodenal branches, and renal artery (curved arrows).

- A 61-year-old female with abdominal pain. Superior mesenteric artery angiogram reveals multifocal areas of irregularity and strictures in the pancreaticoduodenal branches and inferior pancreaticoduodenal pseudoaneurysm (White solid Arrow) with proximal luminal stenosis (White hollow arrow).

- A 61-year-old female with abdominal pain. Successful post-embolization of inferior pancreaticoduodenal pseudoaneurysm with metallic coils using the sandwich technique (White solid arrow). Partially visualized is left common iliac vein stent (Black solid arrow).
DISCUSSION
Aneurysms in the visceral arteries are rare with an incidence of about 2%.[1] PAN is a spectrum of diseases that belong to the pathologic category of necrotizing vasculitis. This spectrum includes Churg-Strauss syndrome, microscopic PAN, Kawasaki disease, rheumatoid vasculitis, Wegener granulomatosis, and hypersensitivity vasculitis. PAN is twice more common in men than women; however, both of our patients were females. It is found in all ages but most commonly in 5th–7th decades. There is a strong association with Hepatitis-B infection and HIV. The most well-known angiographic feature is the presence of microaneurysms in medium to small arteries. The kidneys may be involved in 70–80% of cases; the gastrointestinal tract, peripheral nerves, and skin in 50%; skeletal muscles and mesentery in 30%; and the central nervous system in 10%. PAN should be suspected in patients with fever, weight loss, arthralgia, peripheral neuropathies, abdominal pain, and evidence of multiorgan involvement. PAN has <15% five year survial rate, if its not treated. Survival increases to 80% with steroid treatment and with or without cytotoxic medications.
The diagnosis of PAN is difficult clinically due to the vague symptoms. According to American College of Rheumatology, there are 10 criteria for the diagnosis of PAN, and at least three criteria are required to make the diagnosis. Positive angiographic findings are one of these criteria.[2] Rarely, PAN presents with complications such as RPH related to rupture of renal or other abdominal visceral microaneurysms.
Spontaneous renal hemorrhage (SRH), also called Wunderlich syndrome, is acute onset of non-traumatic sub-capsular and perirenal hematoma. SRH is rare and most commonly due to renal neoplasm, specifically large angiomyolipoma (>4 cm), renal cell carcinoma, and non-neoplastic causes such as vasculitis and infection (less common). PAN is currently the second most common etiology in acute SRH, replacing renal cell carcinoma.[3] SRH is also commonly associated with the anticoagulant medication, bleeding diathesis or inpatient with end-stage renal disease on hemodialysis.[4] SRH is often life-threatening due to non-specific symptomatology at a presentation such as abdominal pain, nausea, vomiting, headache, fever, weight loss, anemia, and microscopic hematuria. Some patients present with acute flank or abdominal pain, palpable flank mass, and hypovolemia called the Lenk’s triad.
One of our patients presented with chronic RLQ abdominal pain, nausea, vomiting, and anterior chest pain for 3 days with CT scan revealing hemorrhage in right anterior pararenal space. The other presented with acute RLQ abdominal pain, diaphoresis, and hypotension with CT scan revealing fractured right kidney with pericapsular hematoma and multiple hepatic and splenic infarcts.
Imaging
CTA is the initial modality of choice. Due to an increase in abdominal angiography, some asymptomatic cases are diagnosed by angiography. Knowledge of the angiographic findings is of importance in diagnosing those. The most common angiographic findings are aneurysms, ectasia, and stenosis in 40–90% patients. Other findings include irregular narrowing of the arteries and pseudoaneurysms. However, these findings are not specific as other vasculitis such as rheumatoid vasculitis, Churg-Strauss syndrome, and systemic lupus erythematous may have similar findings. Thus, clinical evaluation is necessary to confirm the diagnosis.[5] Embolization of the bleeding aneurysms is the treatment of choice. Prophylactically large aneurysms (>2 cm) may be embolized due to the risk of rupture.[6]
Treatment
Transarterial angioembolization of the pseudoaneurysms is now the preferred treatment modality of choice as it is safe, effective, and less invasive and can be both diagnostic and therapeutic. Usually, visceral artery aneurysms require treatment only when the size is >2 cm, which are symptomatic or show interval growth.[6] However, pancreaticoduodenal aneurysms are associated with a high risk of rupture and require immediate treatment with embolization or surgery. Transarterial embolization involves radial or femoral access using 4 or 5 Fr sized arterial sheath with selective catheterization of involved arteries performed using Cobra, or Shepherd hook-shaped 4–6 Fr sized catheters, with microcatheters (2.8 Fr) used for super selective embolization to reduce morbidity and the size of infarcted area.[4,7]
The embolization procedures are performed under moderate sedation or general anesthesia. The choice of agent depends on the site of embolization. Metallic coils (0.035 in or 0.018 in pushable Vortx™ microcoils by Boston Scientific Corporation, Massachusetts, USA and Tornado™ by Cook Medical LLC, Indiana, USA) are commonly used to induce permanent proximal occlusion. These are relatively easy to use and can be retrieved if non-targeted. Sometimes, vascular plugs (Amplatzer™, St. Jude Medical Inc., New Jersey, USA) are used to induce permanent proximal occlusion. They consist of thrombogenic polyester filaments in the detachable nitinol cage. They are used for large vessel embolization with high flow state such as arteriovenous fistulas or aneurysms (splenic or renal).[8]
Complications
Transcatheter arterial embolization is a relatively safe procedure with low complication rates compared to surgical procedures. The non-specific complications of embolization are related to procedure rather than embolization and include groin hematoma, arterial dissection or thrombosis, contrast reactions, and contrast-induced nephropathy.[8,9] The specific embolization related complications include non-targeted embolization causing infarctions in kidney, liver, spine, and bowel or pulmonary embolism.[8] Coil migration can occur but can be removed using snares. Post-embolization syndrome can occur due to necrosis of the masses causing low-grade fever, pain, vomiting, and leukocytosis and can be reduced with pre- and post-embolization corticosteroids.[7,10]
CONCLUSION
PAN has a vague clinical presentation and is clinically occult. Patients may be diagnosed while getting investigated for some other causes of abdominal pain wither with CT scan or during DSA for some other causes. The knowledge of angiographic findings, identification of the aneurysms and narrowing of vessels increases the specificity of diagnosis of PAN, in patients without overt clinical disease. Angiography can play a crucial role, not only in diagnosis but also in the appropriate management. The emergency physician and the radiologist should be aware of the findings and should be able to correlate the imaging findings with pathology to avoid life-threatening complications like RPH.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Polyarteritis nodosa-induced pancreaticoduodenal artery aneurysmal rupture. Int J Angiol. 2015;24:63-6.
- [CrossRef] [PubMed] [Google Scholar]
- The American college of rheumatology 1990 criteria for the classification of polyarteritis nodosa. Arthritis Rheum. 1990;33:1088-93.
- [CrossRef] [Google Scholar]
- Changing etiology and management patterns for spontaneous renal hemorrhage: A systematic review of contemporary series. Int Urol Nephrol. 2017;49:1897-905.
- [CrossRef] [PubMed] [Google Scholar]
- Renal artery embolization-indications, technical approaches and outcomes. Nat Rev Nephrol. 2015;11:288-301.
- [CrossRef] [PubMed] [Google Scholar]
- Polyarteritis nodosa: Spectrum of angiographic findings. Radiographics. 2001;21:151-9.
- [CrossRef] [PubMed] [Google Scholar]
- Hybrid surgery to treat multiple visceral aneurysms secondary to polyarteritis nodosa. Vasc Specialist Int. 2018;34:35-8.
- [CrossRef] [PubMed] [Google Scholar]
- Renal artery embolization. Semin Intervent Radiol. 2011;28:396-406.
- [CrossRef] [PubMed] [Google Scholar]
- Transcatheter arterial embolization in patients with kidney diseases: An overview of the technical aspects and clinical indications. Korean J Radiol. 2010;11:257-68.
- [CrossRef] [PubMed] [Google Scholar]
- Failure rate and complications of angiography and embolization for abdominal and pelvic trauma. J Trauma Acute Care Surg. 2012;73:1208-12.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of postembolization syndrome after complete renal angioinfarction: A single-institution experience over four years. Scand J Urol. 2014;48:245-51.
- [CrossRef] [PubMed] [Google Scholar]







