Translate this page into:
Refractory periocular amblyogenic hemangiomas in childhood: Is there a role for embolization?

*Corresponding author: Andres R Plasencia, MD, Department of Interventional Neuroradiology, Clinica Internacional de San Borja, Lima, Peru. andresplasencia2000@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Plasencia AR, Salini RH, O’Higgins TV. Refractory periocular amblyogenic hemangiomas in childhood: Is there a role for embolization? Am J Interv Radiol 2022;6:13.
Abstract
Objectives:
The aim of the study was to evaluate the role of transarterial embolization (TAE) as a therapeutic modality for potentially amblyogenic periocular infantile hemangiomas (POIH) resistant to medical treatment.
Material and Methods:
We retrospectively analyzed the clinical, angiographic, and imaging data of four patients who underwent TAE as a pre-operative adjunct for POIH causing obstruction of the visual axis.
Results:
Rapid tumor shrinkage with early opening of the visual axis was achieved in all four POIHs. No complications occurred. The best results were achieved in non-congenital infantile hemangiomas and in the proliferative phase of the tumors.
Conclusion:
Our short series demonstrated that TAE may be a salvage treatment to unblock the visual axis before surgery or as a first-line definitive therapy for medically refractory and potentially amblyogenic POIHs.
Keywords
Amblyopia
Embolization
Hemangioma
Periocular
Treatment
INTRODUCTION
Infantile hemangioma (IH) is the most common tumor occurring in childhood, appearing in the 1st weeks of life and growing rapidly to reach maximal size by the age of 6–12 months.[1,2] Involution can begin by the age of 1 year and continue over the course of 4–6 years.[1] After involution, large and superficial facial IHs often leave disfiguring scars.[1] Rarely, an IH is fully developed at birth (congenital hemangioma).[3] An estimated 10% of IHs will not involute spontaneously and will require therapy. Conventional medical treatment with propranolol or corticosteroids leads to remission in most IHs. However, 10–20% of IHs may be resistant to this treatment.[4-6]
Surgery may be prescribed in IHs occurring in critical locations, such as the upper respiratory airway or the mouth, throat, eye, or external ear canal, causing respiratory or cardiac failure, masticatory or swallowing problems, potential amblyopia, or auditory loss. Surgery also plays a role in bleeding IHs or when IHs are associated with Kasabach-Merritt syndrome. Aesthetic deformity in large facial IHs warrants excision to avoid psychological sequelae, although there is a risk of disfiguring post-operative scar.[4,5,7,8]
Pre-operative transarterial embolization (TAE) may be prescribed to prevent excessive blood loss and to shorten operative time.[7-9] Laser therapy is used to treat ulcerated and scarring from IHs that have involuted.[10]
Rare reports exist of TAE being used as a single treatment for maxillofacial IH.[11-14] Since 1996, we have performed pre-operative TAE for periocular hemangiomas. Herein, we report our experience with TAE as a first-line, minimally invasive treatment for visually limiting periocular infantile hemangioma (POIH) resistant to medical treatment.
MATERIAL AND METHODS
Between July 2001 and January 2019, four children with POIHs were referred to our service for TAE. Their ages ranged from 4 to 14 months and they had received corticosteroid therapy using escalating doses of prednisone or prednisolone 2–4 mg/kg/d (cases 1–3) or propranolol 1–3 mg/kg/d (case 4) for 2 months without clinical response. TAE was indicated before surgical resection due to its large volume, causing aesthetic deformity and, above all, because it caused obstruction of the visual axis with the risk of leading to deprivational amblyopia. All of our four patients had clinical and angiographic criteria for hemangioma. None had histological study. The parents had provided consent for each patient. Their clinical files, photographs, and angiographies were studied, and the findings described.
Embolization technique
After receiving informed consent and under intubation and general anesthesia, a 5F and 10 cm-long introducer sheath (Terumo Interventional Systems, Tokyo, Japan) was installed in the right femoral artery using the Seldinger technique. Under fluoroscopy, a 5F Envoy guiding catheter (Johnson and Johnson, New Brunswick, NJ, USA) was placed in the external carotid artery (ECA) in the side of the tumor. The angiograms assessed the dominant feeders involved and confirmed the multilobulated, “cotton wool” appearance and flow patterns of the tumor as the absence of an arteriovenous shunt. Under road-mapping using the coaxial technique, a Renegade Hi Flo microcatheter with a Transend 14 micro guidewire (both from Boston Scientific, Natick, MA, USA) was advanced to reach the feeders distally to avoid reflux to normal vessels as confirmed by digitally subtracted micro-angiography. In all four of our cases, PVA particles sized 250 to 300 microns (Cook Inc. Bloomington, IN, USA) were injected super selectively until obliteration of the arterial supply was achieved.
Our fluoroscopic rate was set to 1–2 images per second and we used the lowest radiation exposure possible. Once the maximal embolization was reached or reflux was obtained, we performed post-procedural angiograms. The catheters were removed and hemostasis with manual compression of the puncture site in the groin was done. Once alert, patients were sent to a recovery unit for clinical and neurological monitoring for 2 h, after which they were transferred to the pediatric ward. Embolization was given as a single session in all four patients.
Clinical management after TAE
Patients were clinically assessed and managed for 1–2 days and discharged to home. Analgesics were prescribed in case of pain. No other medication was given. Clinical follow-up was scheduled at 2 weeks and then monthly, if feasible, for up to 1 year. Photographs were taken to document any changes.
[Table 1] summarizes the patient demographics, clinical, and angiographic features of the tumors, as well as embolization and clinical results. An extensive review of the English-language literature was conducted using the PubMed database from 1965 to the present for pertinent papers on embolization of POIHs.
| Case | Age/Sex | Location/% Hemifacial extent | Tumor Phase/Type | Prior Treatment | Symptoms | Arterial Supply | # of TAEs (if≥2/Obliteration Rate) | Tumor shrinkage at 3-6 mo. | Symptom Resolved | Post TAE Complications |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6 m/M | Lt. Nasogenian groove 60% | NICH | C | AD, Pain, visual axis and nasal obstruction | IM, F | 2/ 100% | 60% | Y (further surgery) | None |
| 2 | 6 m/M | Lt lower eyelid, cheek 50% | NICH | C | AD, Pain, Visual axis obstruction | F, TF, IM, Ophthalmic | 4/ 90% | 40% | Y | None |
| 3 | 1 y/M | Rt lower eyelid 20% | P | C | AD, Obstruction of Visual axis | F, ST | 100% | ≥95% | Y | None |
| 4 | 1 y/M | Lt eyebrow 10% | P | C, P | AD, Pain, Potential visual axis obstruction | ST, Ophthalmic | 100% | 80% | Y (further surgery) | None |
P: Propranolol, PP: Proliferative Phase, I: Involutive phase, NICH: Non-involutive congenital hemangioma, C: Corticosteroids, IL: Intralesional, AD: Aesthetic deformity, Arterial Supply: F: Facial, TF: Transverse facial, IM: Internal maxillary, AA: Anterior auricular, PA: Posterior auricular, ST: Superficial temporal
RESULTS
Patient ages ranged from 6 to 14 months (mean: 10.5 months; median: 9.5 months). There were two females and two males. Our patients from 1-4 correspond to the figures from 1-4, respectively. In two cases (Patients 1 and 2), the tumors were centered in the nasogenian region and extended to the nose and upper cheek. Another case (Patient 3) involved tumors in both eyelids and the last case involved the eyebrow and partially the upper eyelid (Patient 4). Our cases 1, 2, and 3 completely occluded the visual axis, while the fourth involved only marginal occlusion while showing rapid growth. Our cases 1 and 2 were diagnosed as non-involuting congenital hemangiomas (NICHs). The differentiation of our hemangiomas into congenital and non-congenital hemangiomas was based solely on clinical features since at that time, we did not have available an immunohistochemical assay such as the glucose transporter 1. Our Cases 3 and 4 were IHs in their proliferative phase. The previous failed therapeutic regimens were corticosteroids (Cases 1–3), and propranolol (Case 4).
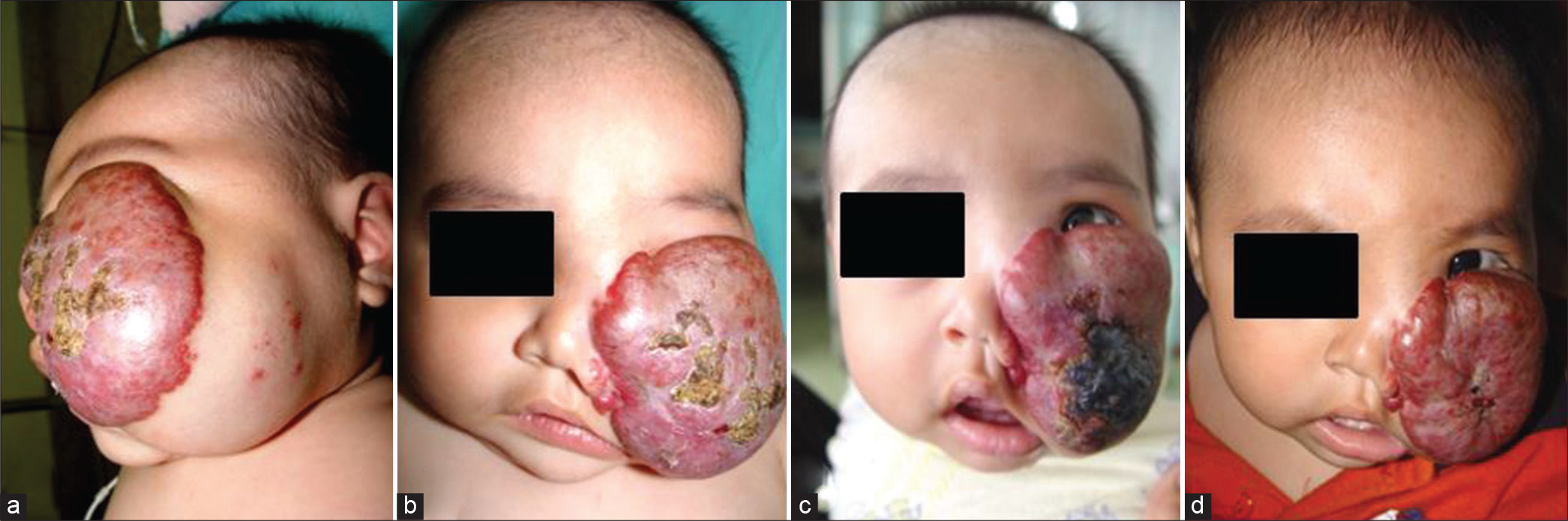
- Case 1 — A 6-month-old male with a large congenital hemangioma involving the left nasogenian groove and part of the cheek, occluding the visual axis. (a and b) Patient’s aspect before transarterial embolization. (c) Two weeks after 65% devascularization. The lesion became partially necrotic, and early shrinkage of the tumor, which cleared the visual axis, was observed. (d) Follow-up 5 months later showed partial involution of 40%. Patient was sent to head-and-neck surgery.
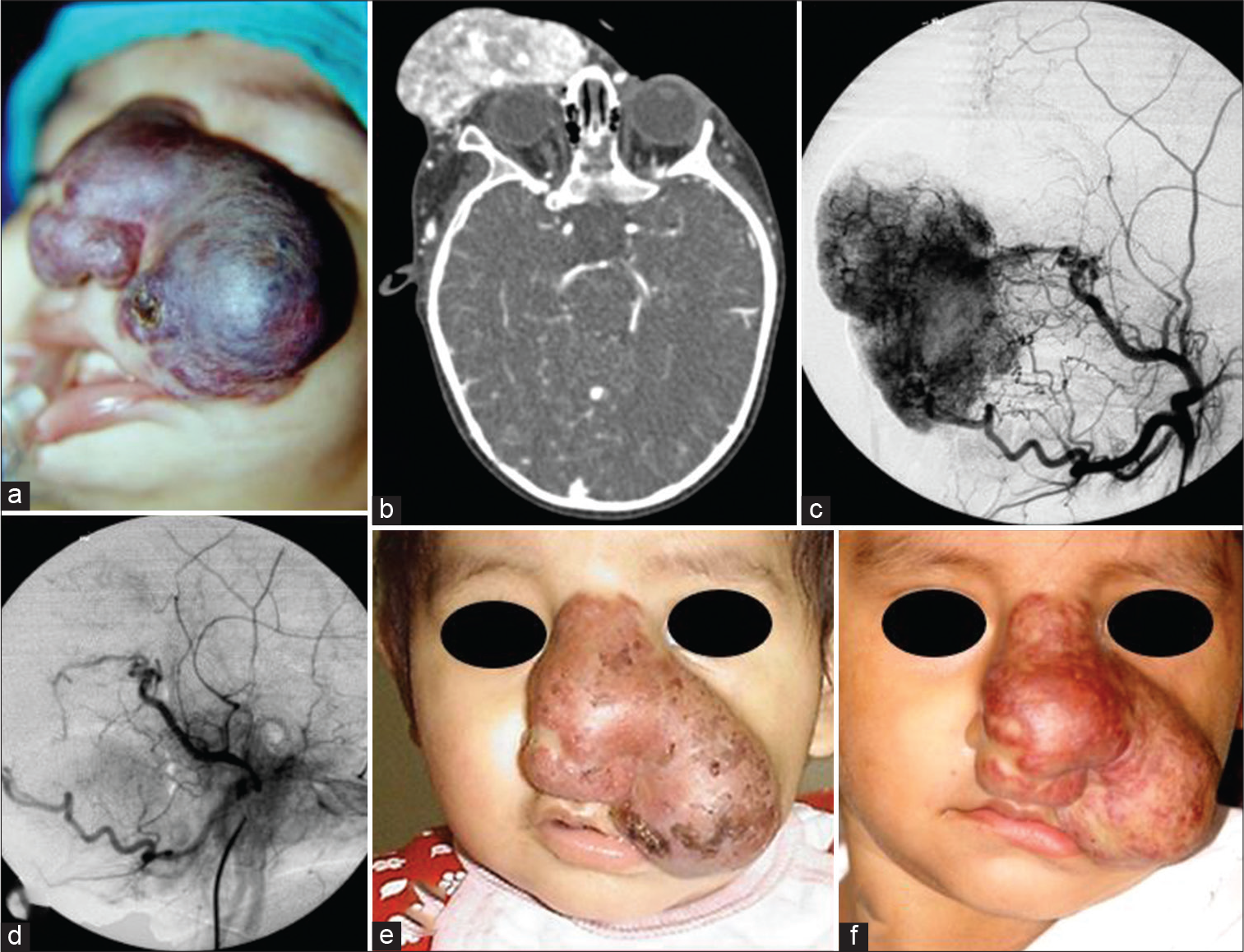
- Case 2 — A 5-month-old girl with a large congenital hemangioma involving the nose and left nasogenian groove occluding the visual axis. (a) Clinical picture at the end of the embolization. Notice the bluish-purple discoloration of the tumor. (b) Pre-transarterial embolization (TAE) CT shows dense and profuse tumor vascularity. (c) ECA angiogram before TAE. (d) After TAE, the hyper vascularity was obliterated at 100%. (e) Shrinkage of the tumor that cleared the visual axis was observed 1 month later. See patient’s photograph at 7 months old. (f) Follow-up at 2 years old showed marked involution. Patient was sent to head-and-neck surgery.
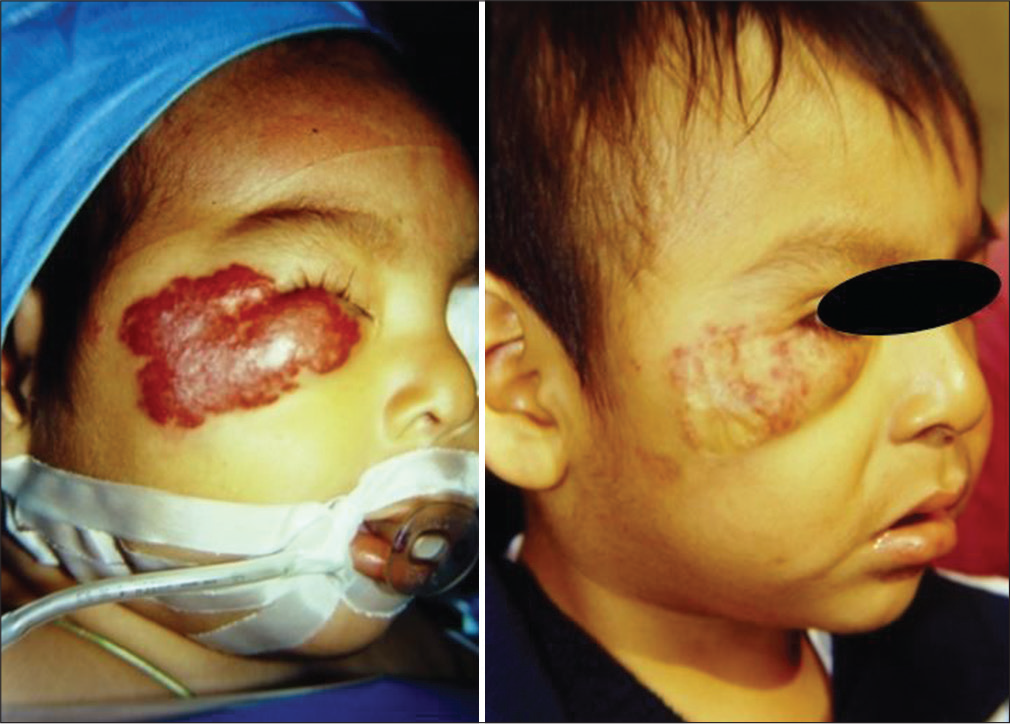
- Case 3 — Pre- and 6 months’ post-embolization of a 14 months old male with a superficial hemangioma of the right malar region and lower eyelid that caused palpebral closure with potential amblyopia. One month after transarterial embolization, the eye aperture was freed. Clinical picture 6 months later showed conspicuous tumor regression. The patient was sent to plastic surgery to consider skin grafting.
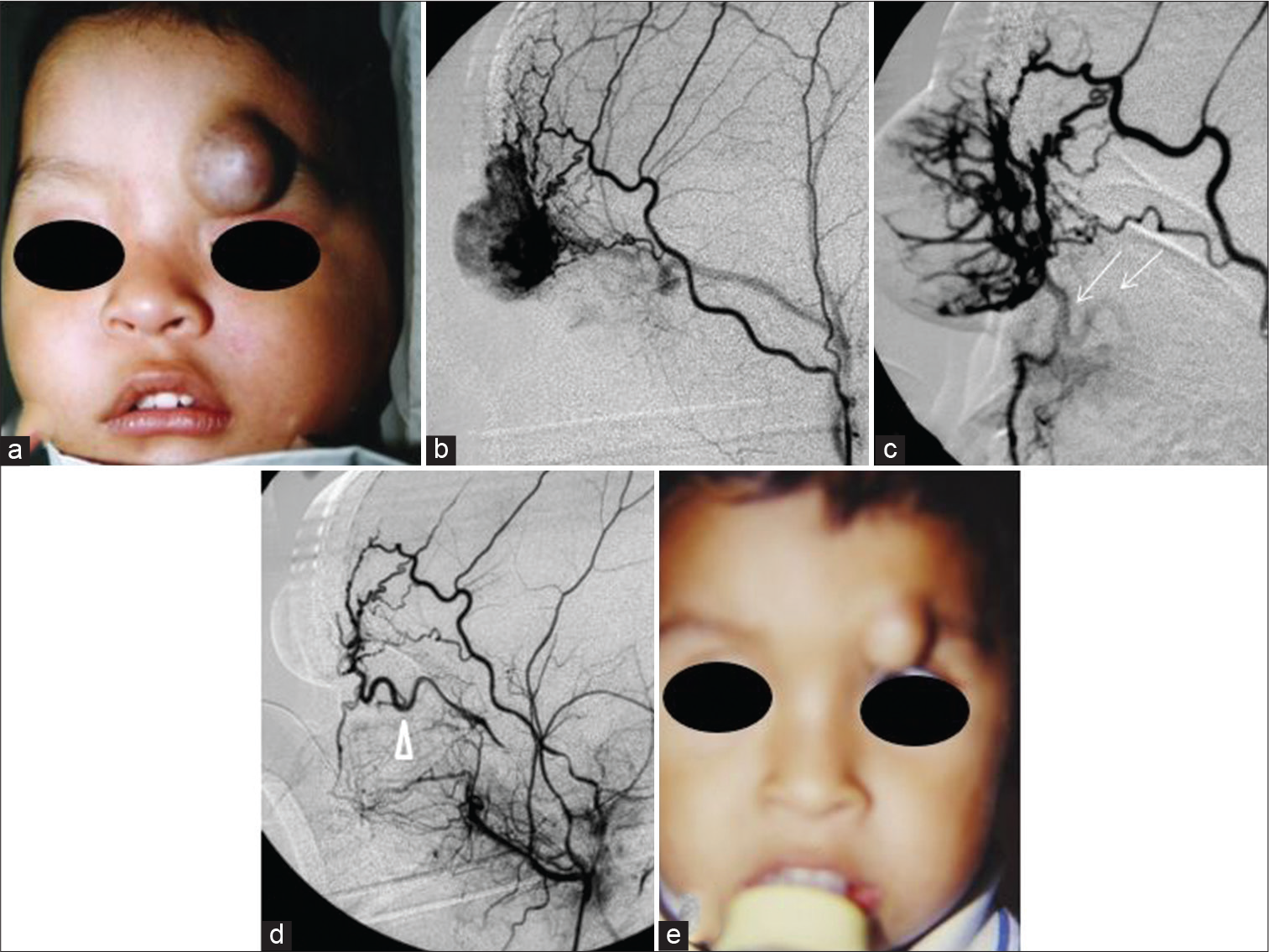
- Case 4 — (a) A 1-year-old male with a rapidly growing left eyebrow infantile hemangiomas before transarterial embolization (TAE). (b) Profuse hypervascularization mainly supplied by the orbitary branch of the superficial temporal artery. (c) A tortuous anastomotic vessel was faintly seen in the final stage of the embolization (thin arrows). (d) Post-TAE angiogram showed a tortuous ophthalmic artery that supplied the tumor as well (arrowhead). It was safely embolized without compromising the central artery of the retina using gentle injections of PVA particles through the orbitary branch of the external carotid artery. (e) Five months after TAE, the tumor was converted to a hypovascularized and fibrofatty lump. The patient was sent to plastic surgery for excision.
Our rationale for performing pre-operative embolization was to decrease intraoperative bleeding. The POIHs were supplied by ECA branches, such as the angular artery of the facial, transverse facial, frontozygomatic, supraorbital, or ophthalmic arteries. The devascularization rate ranged from 75% to 100% (mean: 93.75%). The clinical shrinkages of tumor volume were 50%, 50%, 90%, and 75% (Cases 1, 2, 3, and 4, respectively, mean: 66.25%). The visual axis was completely open a few weeks later in all four cases. In Case 4, the tumor became a small, fibrofatty, and hypovascularized mass. There were no complications related to embolization. All our cases underwent one uneventful TAE session.
We were unable to retrieve the results of the ophthalmologic examinations before and after TAE. A rapid and practically complete opening of the visual axis occlusion occurred in cases 1–3 at 2, 4 and 2 weeks, respectively (mean 2.7 weeks), while in Case 4, it occurred at 12 weeks. Due to significant tumor regression and to the anatomical and functional results achieved early after TAE, surgery was deferred in all patients. Our clinical and angiographic data are summarized in [Table 1].
DISCUSSION
A POIH can affect the visual function in various ways. A lesion deeply seated in the orbit may cause proptosis and corneal exposure problems and/or directly affect the optic nerve leading to its atrophy or involving the extrinsic muscles of the eyeball. Intra- or extra- orbital IHs can push and distort the eye out of alignment, causing strabismus. In such cases, the non-compromised eye will suppress the vision from the deviated eye to prevent diplopia. Conversely, strabismus can not only cause amblyopia, it can also result from amblyopia.
In POIH, the most common cause of amblyopia in induced anisometropia (unequal refracting error between the two eyes), which results from direct mass effect with distortion of the eyeball by the POIH, resulting in astigmatism or myopia. Finally, amblyopia can develop in massive periocular IHs due to mass effect and/or infiltration of the eyelids, resulting in occlusion of the visual aperture and precluding presentation of an image to the brain for a prolonged time (deprivational amblyopia).[15-17] Therefore, there is a narrow therapeutic window of time to resolve the amblyogenic potential of a large POIH that occludes the eye. Williams et al. have shown that children examined and treated for amblyopia before the age of 3 years have better outcomes in terms of visual acuity.[18]
Propranolol is the current treatment of choice for IHs, achieving tumor remission in most patients, leaving few of them with poor or no response.[6] Likewise, surgery for head and neck IHs is limited to small and circumscribed lesions and to incompletely involuted lesions. Surgery for large lesions carries the risks of deforming scars and of producing damage to the facial and trigeminal nerves or to the muscular groups involved in facial expression, along with potential impairment of aesthetics and function, among other complications, particularly in IHs without prior embolization.[19-25]
TAE before surgery has been indicated in select large and richly vascularized IHs to decrease tumor size and intraoperative bleeding.[25-27] However, in potentially amblyogenic POIHs, surgery cannot remove all of the tumor due to risks of massive bleeding and the risk of injury to the extraocular and eyelid muscles.[3,21,24,27] Therefore, in some cases, a partial excision limited to lesions located in the preseptal compartment, leaving in situ sensitive regions for the visual function, may be reasonable. In such cases, the main goal of rapid unblocking of the visual axis may not always be achieved.[21,24]
TAE has been suggested as a stand-alone treatment for maxillofacial IHs, but the supporting evidence is limited to case reports and a few small case series using direct puncture and/or sclerotherapy.[28-30] We found four reports of TAE series with ≥3 cases. Demuth et al. treated three cases with acrylate embolization, achieving moderate shrinkage in all.[11] Two of them developed skin necrosis. Jianhong et al. embolized ten cases using TAE and bleomycin sclerotherapy until they reached a level of surgical manageability. The authors achieved a ≥75% of tumor mass shrinkage without significant or permanent complications. In five of their patients (50%), surgery was not needed.[12]
Patel et al. employed TAE in ten patients suffering from mostly facial NICHs using a wide array of embolic materials, most of which were complimented by direct puncture sclerotherapy in one or two sessions. In eight of their patients (80%), surgery was not needed.[13] Plasencia and O’Higgins reported six large parotid IHs treated with TAE using 300–500 microns PVA particles alone, for which they achieved complete shrinkage and avoided excisional surgery in all cases. One case had a partially reversible hemi-facial hypoesthesia in the side of the tumor. The authors remarked on the efficacy of ischemia in the shrinking of the IH. In all four papers, no recurrences were found in the follow-up period.[14] Therefore, TAE, with or without additional sclerotherapy, may be a reliable therapeutic choice in large POIHs, either alone or as a pre-operative and adjunct measure.
Few papers address TAE as a first line treatment for POIHs. In 1978, Kennedy was the first to report a case of POIH and advocate use of TAE as a definitive treatment.[16] Burrows et al. reported four palpebral IHs with complete (two cases) or partial (two cases) visual occlusion. One had post-procedural excisional surgery. The remaining three cases experienced partial POIH involution and vision was preserved in one case. They did not report complications.[31] Recently, Hadrawi et al. treated one case of POIH using TAE and propranolol obtaining slow regression. Superselective TAE with liquid agent PHIL obtaining 70% of obliteration of its arterial supply, resulting in noticeable reduction in tumoral volume, allowing opening of the affected eye starting at 1-week postoperatively. However, we preferred 250–300 PVA particles instead of liquids as embolic agents due to the fear of liquids to reach normal arteries such the ophthalmic through its anastomotic networks.[32]
The four POIHs that we report included two large POIHs involving the nasogenian groove, cheek, and lower eyelid, and two mid-sized tumors, one of which involved the malar region and eyelids, while the other involved the eyebrow, causing progressive ptosis. Cases 1–3 [Figures 1-3] had completely obstructed one ocular visual field. The visual axis was opened rapidly and completely in all cases, except in Case 1, in which the visual axis was opened partially but significantly [Figure 1]. Amblyopia could have potentially been avoided in cases 1–3 [Figures 1-3]. The two larger POIHs (cases 1 and 2) were sent for delayed elective excisional surgery, while the other two tumors only needed cosmetic surgery. Once again, we were unable to retrieve ophthalmologic data post-TAE.
Even given the absence of complications in our four patients, we must emphasize that selective arteriography and super-selective micro-catheterization is difficult in infants whose vessels are short, thin, and prone to arterial spasm, dissection, and thrombosis. TAE in POIHs should be performed with extreme caution because it can result in serious and potentially fatal complications and, therefore, should be done only by trained teams.[33] External-internal arterial-arterial anastomoses are open in babies and children and embolic material can migrate to the retina and the brain. Injections of corticosteroids and sclerosing agents can thus occlude the ophthalmic and central artery of the retina, producing infarction with amaurosis and/or skin and eyelid necrosis.[34,35]
In our series of four cases of POIHs resistant to medical therapy and treated with TAE, a rapid, almost complete opening of the visual axis was obtained in all cases over a period of 2–4 weeks. These favorable results achieved after TAE were associated with a ≥80 devascularization rate. Our best results occurred when the IH was non-congenital and in the proliferating phase, whereas, in the NICHs, tumor shrinkage was moderate but enough to achieve significant opening of the visual axis. Our results are comparable to those achieved in similar experiences with TAE and sclerotherapy for IHs.[19,20] We must emphasize that, unlike these reports, we only used inert and non-bioactive embolic materials (PVA particles), meaning that ischemia itself may be the final common pathway for IH involution, regardless of the embolic agent used.
This small case series suggests that TAE may be an acceptable therapeutic option for large and richly vascularized POIHs resistant to medical treatment or for which conventional medical treatment is inadequate, in which a rapid opening of the visual axis is needed to prevent deprivational amblyopia while deferring surgery. When the POIH shrinks after TAE, it can be treated with the sclerotherapy necessary to reduce the tumor to its minimum expression to facilitate further definitive excisional and cosmetic surgery, if needed.
CONCLUSION
Our preliminary experience with four potentially amblyogenic POIHs resistant to medical treatment using TAE with PVA particles yielded favorable results, resulting in at least partial tumor shrinkage sufficient to open the visual axis without complications. The best results were associated with non-congenital POIHs in their proliferative phase, in which ≥80% devascularization was achieved. This paper adds to the currently limited literature on TAE for POIH. Early use of TAE in infants and young children with large POIHs may prevent deprivational amblyopia.
Acknowledgment
The experience described in this paper would not have been possible without the teachings and support of Fernando Viñuela, MD.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Congenital hemangiomas: Rapidly involuting and noninvoluting congenital hemangiomas. Arch Facial Plast Surg. 2005;7:307-11.
- [CrossRef] [PubMed] [Google Scholar]
- Management of parotid hemangioma in 100 children. Plast Reconstr Surg. 2004;113:53-60.
- [CrossRef] [PubMed] [Google Scholar]
- A practical guide to treatment of infantile hemangiomas of the head and neck. Int J Clin Exp Med. 2013;6:851-60.
- [Google Scholar]
- Progress in the treatment of infantile hemangioma. Ann Transl Med. 2019;7:692.
- [CrossRef] [PubMed] [Google Scholar]
- The surgical management of infantile hemangiomas. Otolaryngol Clin North Am. 2018;51:125-31.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical treatment of infantile hemangioma in a multidisciplinary vascular anomalies clinic. Int J Pediatr Otorhinolaryngol. 2011;75:1271-4.
- [CrossRef] [PubMed] [Google Scholar]
- Extensive facial vascular malformations and haemangiomas: A review of the literature and case reports. J Craniomaxillofac Surg. 1997;25:335-43.
- [CrossRef] [PubMed] [Google Scholar]
- Hemangiomas of infancy. J Am Acad Dermatol. 2003;48:477-93. quiz 494-6
- [CrossRef] [PubMed] [Google Scholar]
- Complications of embolization treatment for problem cavernous hemangiomas. Ann Plast Surg. 1984;13:135-44.
- [CrossRef] [PubMed] [Google Scholar]
- Transcatheter arterial embolization in the treatment of extensive maxillofacial hemangioma in children. World J Surg. 2005;29:1550-6.
- [CrossRef] [PubMed] [Google Scholar]
- Angiographic and clinical features of noninvoluting congenital hemangiomas. AJNR Am J Neuroradiol. 2019;40:845-8.
- [CrossRef] [PubMed] [Google Scholar]
- Large parotid and cheek hemangiomas refractory to medical treatment: Is there a role for embolization? Am J Interv Radiol. 2020;4:21.
- [CrossRef] [Google Scholar]
- Ophthalmic issues in hemangiomas of infancy. Lymphat Res Biol. 2003;1:321-30.
- [CrossRef] [PubMed] [Google Scholar]
- Arterial embolization of orbital hemangiomas. Trans Am Ophthalmol Soc. 1978;76:266-77.
- [Google Scholar]
- Periocular capillary hemangiomas: Indications and options for treatment. Middle East Afr J Ophthalmol. 2010;17:121-8.
- [CrossRef] [PubMed] [Google Scholar]
- Amblyopia treatment outcomes after screening before or at age 3 years: Follow up from randomised trial. BMJ. 2002;324:1549-53.
- [CrossRef] [PubMed] [Google Scholar]
- Resolution of astigmatism after surgical resection of capillary hemangiomas in infants. Ophthalmology. 1997;104:1102-6.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical excision of periorbital capillary hemangiomas. Ophthalmology. 1994;101:1333-40.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical treatment of periocular hemangiomas: A single-center experience. Plast Reconstr Surg. 2007;119:1553-62.
- [CrossRef] [PubMed] [Google Scholar]
- Resection of amblyogenic periocular hemangiomas: Indications and outcomes. Plast Reconstr Surg. 2010;125:274-81.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical intervention of periocular infantile hemangiomas in the era of β-blockers. Ophthalmic Plast Reconstr Surg. 2020;36:70-3.
- [CrossRef] [PubMed] [Google Scholar]
- Combined management of a disfiguring facial hemangioma by endovascular embolization and surgical excision: Case report. J Pediatr Surg Case Rep. 2019;48:101273.
- [CrossRef] [Google Scholar]
- Segmental hemangioma of infancy complicated by life-threatening arterial bleed. Pediatr Dermatol. 2009;26:469-72.
- [CrossRef] [PubMed] [Google Scholar]
- Management of a large congenital hemangioma obstructing visual axis: A case report and review of literature. Ophthalmic Plast Reconstr Surg. 2019;35:e154-7.
- [CrossRef] [PubMed] [Google Scholar]
- Selective embolization and resection of a large noninvoluting congenital hemangioma of the lower eyelid. Ophthalmic Plast Reconstr Surg. 2009;25:111-4.
- [CrossRef] [PubMed] [Google Scholar]
- How to use bleomycin A5 for infantile maxillofacial haemangiomas: Clinical evaluation of 82 consecutive cases. J Craniomaxillofac Surg. 2011;39:482-6.
- [CrossRef] [PubMed] [Google Scholar]
- Pingyangmycin sclerotherapy for infantile hemangiomas in oral and maxillofacial regions: An evaluation of 66 consecutive patients. Int J Oral Maxillofac Surg. 2011;40:246-51.
- [CrossRef] [PubMed] [Google Scholar]
- Intralesional bleomycin for the treatment of periocular capillary hemangiomas. Indian J Ophthalmol. 2012;60:326-8.
- [CrossRef] [PubMed] [Google Scholar]
- Urgent and emergent embolization of lesions of the head and neck in children: Indications and results. Pediatrics. 1987;80:386-94.
- [CrossRef] [PubMed] [Google Scholar]
- Early vascular embolization of large orbital and periorbital infantile capillary hemangiomas; a case report. Am J Ophthalmol Case Rep. 2022;10:101377.
- [CrossRef] [PubMed] [Google Scholar]
- Superficial hemangiomas: Associations and management. Pediatr Dermatol. 1997;14:173-9.
- [CrossRef] [PubMed] [Google Scholar]
- Retinal and choroidal microvascular embolization with methylprednisolone. Retina. 2002;22:382-6.
- [CrossRef] [PubMed] [Google Scholar]
- Eyelid necrosis following intralesional corticosteroid injection for capillary hemangioma. Ophthalmic Surg. 1987;18:103-5.
- [CrossRef] [Google Scholar]







