Translate this page into:
Percutaneous Sacroplasty for Sacral Insufficiency Fractures: Case Series and Review of Presentation, Diagnosis, and Treatment

Corresponding Author: Andrew C. Clark, Department of Radiology, University of Rochester, Rochester, New York 14607, United States. E-mail: andrewc_clark@urmc.rochester.edu
-
Received: ,
Accepted: ,
How to cite this article: Clark AC, Butani D. Percutaneous Sacroplasty for Sacral Insufficiency Fractures: Case Series and Review of Presentation, Diagnosis, and Treatment. Am J Interv Radiol 2019;3(4):1-11.
Abstract
Sacral insufficiency fractures (SIFs) are a cause of debilitating low back pain that is often difficult to diagnosis and manage. The diagnosis of SIF is often delayed due to inaccurately attributing symptoms to spondylosis, which is a commonly present in the elderly population where SIFs are most prevalent. Historically, treatment consisted of medical management and open reduction internal fixation reserved for severe cases. However, percutaneous sacroplasty has emerged as a minimally invasive treatment option which provides early pain relief without significant complications. The objective of this article is to raise awareness of SIFs and percutaneous sacroplasty as an effective and safe treatment method.
Keywords
Sacroplasty
Sacral insufficiency fractures
Interventional radiology
Osteoporosis
INTRODUCTION
Sacral insufficiency fractures (SIFs) were first described by Lourie in 1982.[1] Now, decades later, the exact incidence of SIFs remains unknown, while the prevalence is estimated at 1.8%.[2,3] The unknown prevalence of SIFs is likely due to the lack of clinical awareness and underdiagnosis. 90% of SIFs occur in the female population over the age of 60.[4,5] The most common predisposing condition for SIFs is postmenopausal osteoporosis, although secondary causes of osteoporosis such as corticosteroids or radiation also increase the risk.[2] The diagnosis of SIF is confirmed with magnetic resonance imaging (MRI) or scintigraphy, with a preference for MRI due to its higher specificity and greater anatomical characterization.[6]
Before the advent of percutaneous sacroplasty in 2001, most of the SIFs were treated with medical management alone and severe cases treated with surgical fixation.[7] Since the inception of sacroplasty, there has been growing evidence of this technique’s potential for significant pain reduction, improved mobility, and decreased opioid dependence and with minimal risk.[8-12]
Our experience with percutaneous sacroplasty supports the procedure as an effective and safe method to significantly reduce pain in patients suffering from SIFs.
ETIOLOGY OF SIFs
Stress fractures can be classified as either fatigue or insufficiency. A fatigue fracture occurs due to abnormal stress on normal bone, such as repetitive stress seen in long-distance runners or military personnel with frequent marching. Insufficiency fractures occur due to normal stress on abnormal bone, with postmenopausal primary osteoporosis being the most common underlying etiology of SIFs.[2] Secondary causes of osteoporosis should be considered in young or male patients presenting with SIF. There are a wide variety of disease processes and medications that can cause secondary osteoporosis including rheumatoid arthritis, corticosteroids, and prior radiation therapy. Dual-energy X-ray absorptiometry is used for the evaluation of bone mineral density (BMD) and performed by imaging the proximal femur, distal radius, and lumbar vertebra.[13] The World Health Organization defines osteoporosis as a BMD of 2.5 standard deviations (SDs) below controls and osteopenia as 1.5–2.5 SD below controls.[14]
PRESENTATION OF SIFs
The presentation of SIF is generally insidious with symptoms consisting of lower back pain, with radiation to the buttock or groin.[15] Pain is often exacerbated by activity and improved with rest. Two-thirds of all SIFs have no history of inciting trauma.[4] If the fracture involves the sacral neuroforamina or central canal, there can be sphincter dysfunction or lower extremity paresthesia. Although neurological complication is rare, occurring in only 2% of reported SIF cases.[16]
DIAGNOSIS OF SIFs
The diagnosis of SIFs begins with a thorough physical examination to localize the pain to the sacrum. Then, dedicated imaging of the sacrum can be performed. Per the American College of Radiology appropriateness criteria (AC), when there is a suspicion of SIF, radiographs should be the initial imaging examination performed.[6] If the radiographs are inconclusive and suspicion remains high for SIF, then MRI should be performed and is considered the gold standard for diagnosis.[6] If MRI is not available, bone scintigraphy is an acceptable alternative.[6] Computed tomography (CT) is generally used for characterization if intervention is being considered.
Physical examination
Physical examination includes sacral palpation, focused neurological examination, and special orthopedic physical examination tests. Direct sacral palpation may elicit pain but is a non-specific finding. Neurological examination includes evaluating anal tone and the Achilles reflex. The special physical examination tests stress the sacrum and will illicit pain if SIF is present.[2]
Flexion abduction external rotation (FABER) test
The FABER test is performed with the patient in the supine position. One leg is flexed at the knee, externally rotated, and abducted, which places the patient in the “Figure of 4.” The examiner then places downward pressure on the flexed knee and contralateral anterior superior iliac spine toward the floor.
Gaenslen’s test
Gaenslen’s test is performed with the patient supine. One leg flexed maximally at both the knee and hip. The contralateral extended beyond the table to promote extension at the hip. The examiner then exerts downward pressure on both the knees toward the floor.
Squish test
The squish test is performed with the patient supine. The examiner exerts medial pressure into both anterior superior iliac spines, which places traction lateral traction on the sacrum. The examiner then exerts lateral pressure into both anterior superior iliac spines, which compresses the sacrum.
Laboratory evaluation
The majority of laboratory testing is used for determining secondary causes of osteoporosis and not for the direct diagnosis of SIF. However, serum alkaline phosphatase is a non-specific marker of SIF as values will be elevated levels in the setting of bone formation.[15]
Imaging
The American College of Radiology AC provides guidance for imaging in various clinical scenarios, rating each imaging modality on a 1–10 scale. The AC gives a strong recommendation for radiography as the initial study when SIF is suspected, rated at 9. If initial radiographs are negative, then the MRI (rating 9), CT (rating 7), and/or bone scintigraphy (rating 6) can be performed.[6]
Radiography
The most common appearance of SIF on radiographs is sclerosis of the sacral wing with or without cortical break. The sensitivity for the detection of SIF on radiography is poor, estimated at 20–38%.[4] This poor sensitivity is can be due to a combination of several factors including underlying osteopenia, fecal material, bowel gas, copious soft tissue, or X-ray occult fracture.[6,17] An example of a right-sided SIF on radiography is demonstrated in Figure 1.
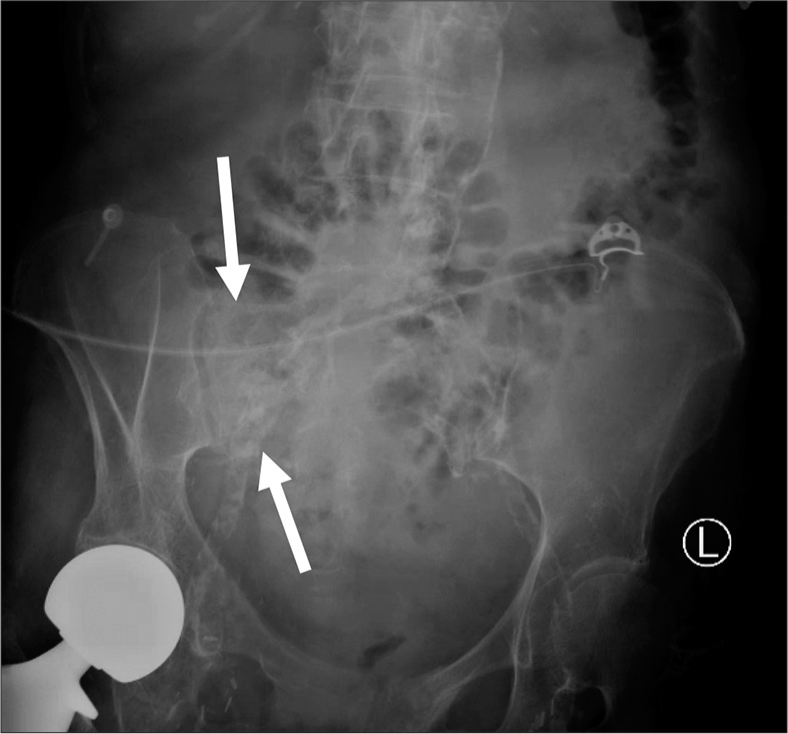
- A 68-year old male with known sacral insufficiency fracture. Anterior posterior radiograph of the pelvis demonstrates subtle sclerosis of the right sacral ala.
CT
CT has improved sensitivity for the detection of SIFs, compared to radiography, estimated at 60–75%.[18] CT provides additional bony detail which is helpful if invasive intervention is being considered as a fracture line extending into the sacral foramina could act as an undesirable pathway for cement.[19] Figure 2 demonstrates an example of the right-sided SIF by CT.
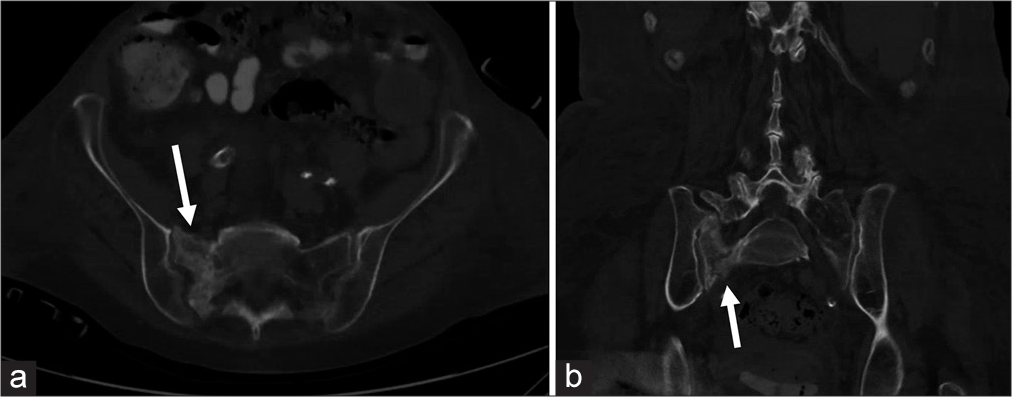
- A 68-year-old male with known sacral insufficiency fracture. (a) Axial computed tomography with (b) coronal reformat of the same patient in Figure 1 reveals sclerosis of the right sacral ala with fracture line and cortical break.
MRI
MRI is considered the gold standard for SIFs, with a sensitivity of nearly 100%.[18] T2 Short tau inversion recovery (STIR) is the most sensitive sequence for the detection of early SIF. Bone marrow edema will appear as areas of hyperintensity on STIR sequences and hypointensity in T1-weighted sequences, in one or both of the sacral alae. A hypointense fracture line will be seen within the area of edema 93% of the time.[6,18] Figure 3 demonstrates an example of the right-sided SIF by STIR MRI.
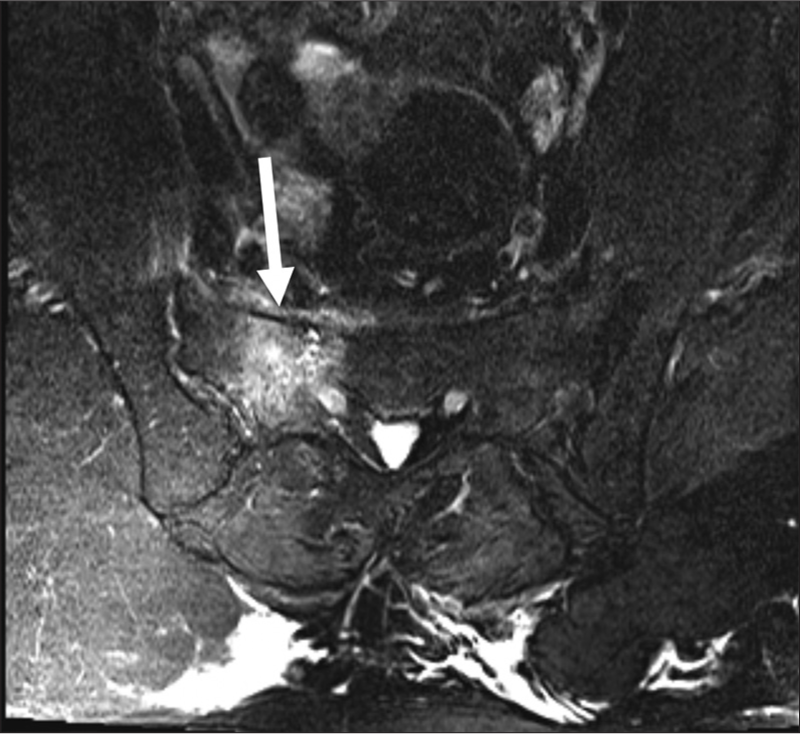
- A 77-year-old female with known sacral insufficiency fracture. T2 short tau inversion recovery (STIR) axial image showing bone marrow edema in the right sacral wing, diagnostic of a sacral insufficiency fracture. STIR imaging sequence is exquisitely sensitive for the detection of bone marrow edema.
Bone scintigraphy
Before MRI, technetium-99m nuclear medicine bone scintigraphy was the gold standard for the detection of SIF, with an estimated sensitivity for the detection of stress fractures at 93%.[20] The classic appearance of SIF on bone scan is the H-pattern, which is an uptake in both sacral alae with a horizontal connecting strut, although this is only seen in around 40% of cases.[4] Scintigraphy lacks specificity as uptake will be present not only with SIF but also with metastatic lesions, osteomyelitis, sacroiliitis, and physiologic sacral iliac (SI) joint uptake.[21] Figure 4 demonstrates a nuclear medicine bone scintigraphy posterior planar image with the classic H-shaped uptake of a Denis 3 fracture.
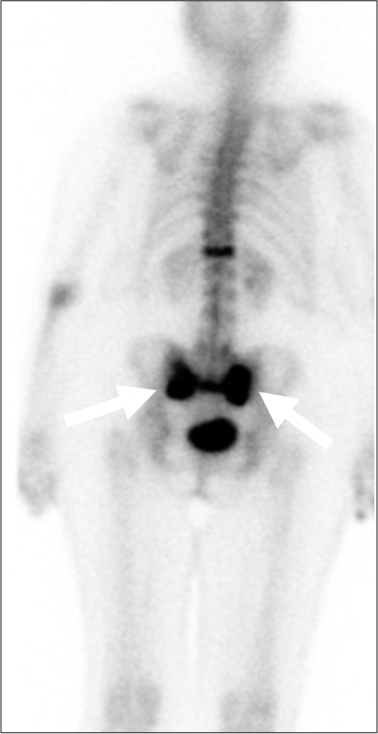
- A 72-year-old female with known sacral insufficiency fracture. Posterior planar bone scintigraphy. demonstrates the classic H-shaped uptake, seen in a Denis type 3 sacral fracture.
CLASSIFICATION OF SIFs
The Denis Classification is a commonly used system for the classification of traumatic sacral fractures created in 1988 and can be adapted for the classification of SIFs.[22] The Denis classification demarcates three vertical zones of the sacrum. A zone 1 fracture involves one or both sacral alae, with no involvement of the sacral neuroforamina or midline structures. Zone 2 fractures involve the neuroforamina but spare the midline. Zone 3 fractures involve the midline sacrum and can be further subclassified depending on the presence of angulation, displacement, and commutation. A summary of the Denis classification is described in Table 1. A A three-dimensional volume rendered computed tomographic image of a sacrum with the zones of the Denis classification is demonstrated in Figure 5.
| Denis classification | |
|---|---|
| The Denis classification separates sacral fractures into three zones. | |
| Zone 1: | Fracture involving one or both sacral alae. No involvement of neuroforamina or central canal. |
| Zone 2: | Fracture involving neuroforamina. Central canal is spared. |
| Zone 3: | Fracture involving central canal and is further divided into four subtypes. |
| Type 1: | Kyphotic angulation without translation |
| Type 2: | Kyphotic angulation with translation of distal fragment. |
| Type 3: | Kyphotic angulation with complete offset of distal fragment. |
| Type 4: | Comminuted S1 segment. |
| Morphologic descriptions of zone 3 fractures: H, U, λ, or T shaped |
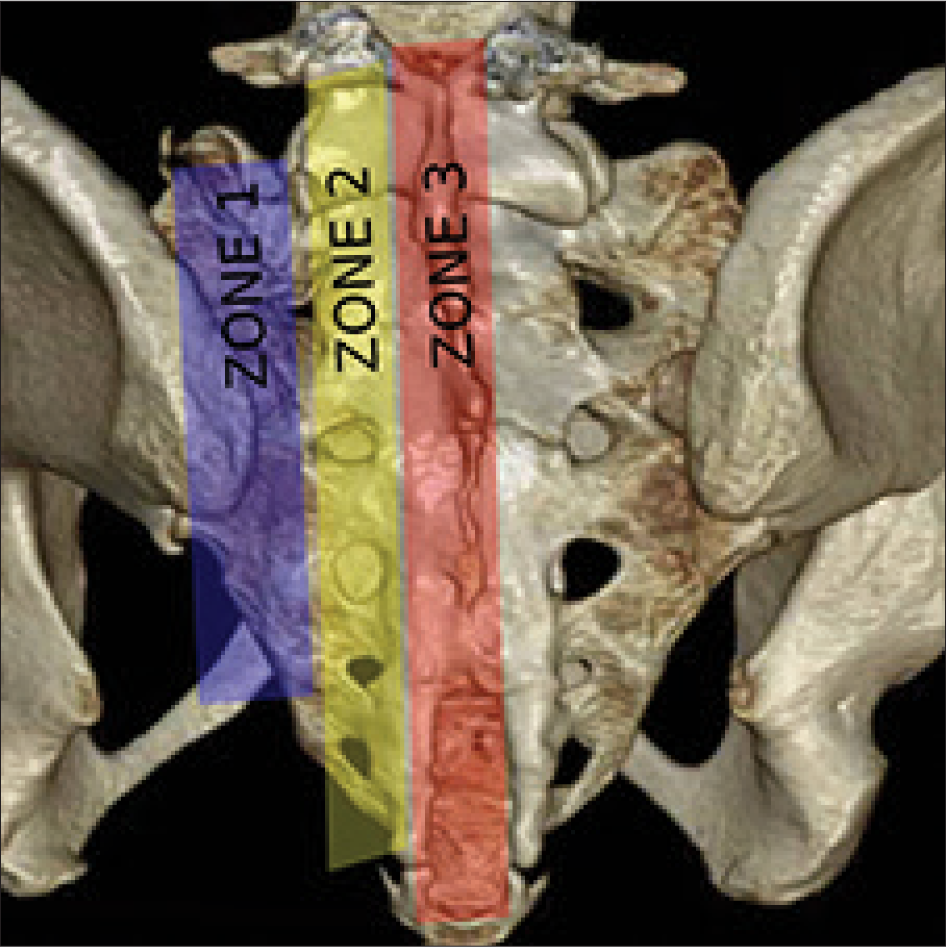
- A three-dimensional volume rendered computed tomographic image of the sacrum with the demarcated zones described in the Denis classification of sacral fractures.
TREATMENT AND MANAGEMENT OF SIFs
Treatment of SIFs consists of non-invasive intervention with or without invasive interventions. Medical management consists of a combination of best rest, physical therapy, analgesia, and bone loss prevention medications. Invasive interventions can be divided into minimally invasive percutaneous sacroplasty and various forms of surgical fixation.
Physical therapy
Classic conservative treatment originally consisted of 3–6 months of bed rest; however, more modern approaches employ early ambulation and weight bearing exercises as this has been shown to induce osteoblastic bone stimulation.[15,23] Early ambulation is also recommended as prolonged bed rest is associated with deep venous thrombosis, pressure ulcers, deconditioning, infection, and negative psychologic impact.[15]
Medications
Medications used in the setting of SIFs are directed at pain control and decreasing further bone loss.
Analgesic medications
Analgesic medications consist of nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids. NSAID use is controversial as there is some evidence that their use may inhibit osteoblastic activity and slow fracture healing.[24] Opioids can provide excellent pain control; however, there is association with increased rate of falls and mortality when used in the elderly.[25]
Vitamin D and calcium
Vitamin D and calcium supplementation should be initiated to slow further bone loss. Recommended daily dosages include 1200–1500 mg calcium and 400–800 IU Vitamin D daily.[14]
Bisphosphonates
Bisphosphonates are one of the most widely used medications to halt the resorption of bone in osteoporosis. Bisphosphonate accumulates in the calcium of bones and has a dual action, of inducing apoptosis in osteoclasts and promotion of osteoblasts.[26] The major bone benefit of bisphosphonates occurs in the first 5 years of use.[15,27] Discontinuation of bisphosphonate use after 5 years may be preferred as there is a causal relationship with prolonged bisphosphonate use and atypical femoral fractures.[28]
Anabolic medications
Anabolic medications include teriparatide and strontium. Teriparatide is a synthetic polypeptide consisting of fragments of parathyroid hormone, which has been shown to improve architecture of both trabecular and cortical bones.[29] The use of teriparatide for the treatment of insufficiency fracture healing is unclear with some studies showing improved healing times and others showing no difference to placebo.[30,31] Strontium ranelate is a salt of element strontium which is nearly identical to calcium and has been shown to both decrease osteoclastic activity and increase osteoblastic activity, therefore improving BMD.[32] Strontium ranelate is not currently approved by the FDA for treatment osteoporosis in the United States but is used in multiple other countries.
Invasive interventions
Invasive interventions consist of percutaneous sacroplasty and various forms of surgical fixation. Sacroplasty is considered a minimally invasive technique as it can be performed under moderate sedation on the same-day outpatient basis. Surgical fixation is more invasive requiring general anesthesia, post-surgical inpatient monitoring, and possible need for future revisions.
Percutaneous sacroplasty
Sacroplasty is a minimally invasive, percutaneous, image-guided technique for the treatment of SIFs and painful sacral metastasis. The procedure involves the injecting of polymethylmethacrylate bone cement through one or more trocar needles in the affected sacral wing. The first documented use of sacroplasty was in 2001 for the treatment of painful sacral metastasis followed by treatment of SIFs in 2002.[7,33,34] Choice of image guidance is operator dependent and can consist of a combination of fluoroscopy, fan beam CT, and cone-beam CT. The two main needle approaches are posterior and long-axis.
Posterior approach sacroplasty technique
A majority of operators prefer a posterior approach due to the similarity to vertebroplasty.[35] The posterior approach is performed with the patient in the prone position. Most commonly, two needles are used per treatment side. Each needle is placed in the S1 and S2 levels to ensure adequate cement delivery. If fluoroscopy is being utilized, the anterior oblique view is obtained by aligning the detector parallel with the L5–S1 joint and ipsilateral SI joint. For S1 sacral ala treatment, the needle is advanced through the posterior cortex en face, 1–2 cm caudal to the superior margin of the sacral ala and 1–2 cm medial to the SI joint.[35,36] On the lateral view, the target position of the needle tip when treating S1 is the intersection of the lines drawn from the corners of S1.[37] Violation of the anterior sacral cortex is the major risk of the posterior approach. Examples of ideal needle placement for the posterior approach are demonstrated in Figure 6a and b.
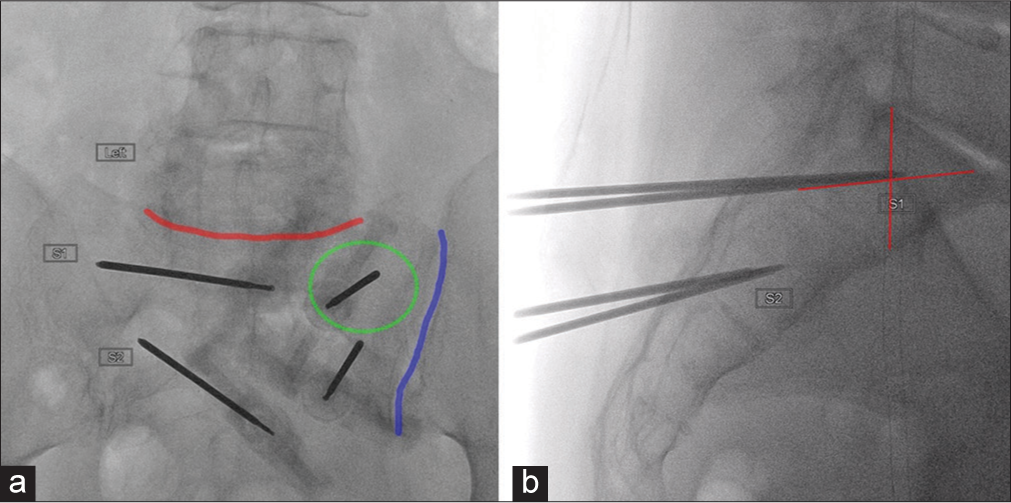
- Ideal fluoroscopic views and needle placement for posterior approach sacroplasty. (a) Anterior oblique view with L5–S1 (red line) and the sacroiliac joint (blue line) aligned with the detector. The needle within the left S1 wing is advanced face (green circle). (b) Target of S1 needle tip at the intersection of lines drawn from the corners of S1.
Long-axis approach sacroplasty technique
The long-axis approach is performed with the patient in the prone position. A needle is advanced from caudal to cranial along the long axis of the sacral wing. The advantages of the long-axis approach are improved cement distribution, only one needle required per side, and decreased chance of anterior cortex violation.[35] The ideal needle entry site is the midpoint between the lower SI joint margin and lateral margin of the S3 foramina, as shown in Figure 7a.[19] The lateral view is used to advance the needle tip to overlie the center of S1 and portrayed in Figure 7b.
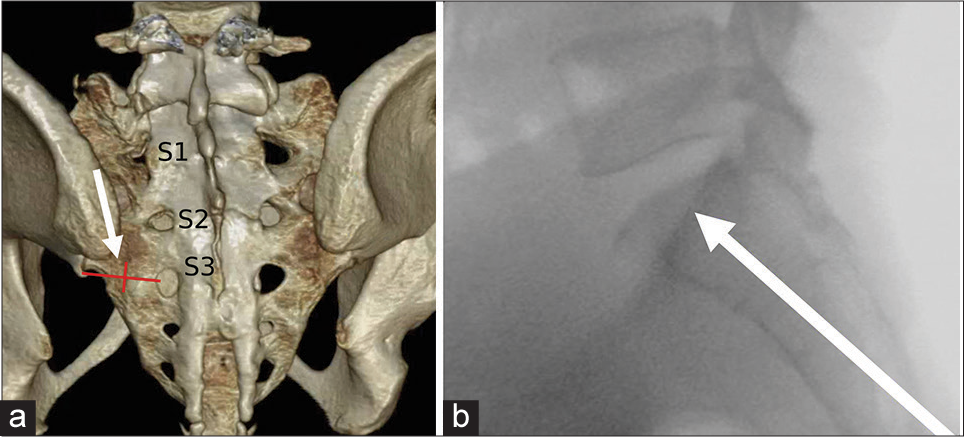
- Ideal needle trajectory for long-axis approach to sacroplasty. (a) A three-dimensional volume rendered computed tomographic image with the target of needle entry at the midpoint between the inferior margin of the SI joint and lateral margin of S3 neuroforamina. (b) Lateral fluoroscopic view with the needle tip in optimal position for cement injection, overlying the center of S1.
Sacroplasty outcomes and safety
There are several studies that suggest sacroplasty permits early symptomatic relief, decreased opioid use, and improved mobility compared to non-invasive intervention.[8,9,11] One prospective study showed a 60% decrease in pain 30 min post sacroplasty, 70% at 2 weeks, 75% at 4 weeks, 80% at 12 weeks, 85% at 24 weeks, and 90% at 52 weeks.[9]
The theoretical risks of sacroplasty are associated with cement leak beyond the sacral cortex. Cement leak into the venous system can result in pulmonary embolism. Leakage of cement into the sacral neuroforamina or central canal could result in neurological compromise. Although with a review of current literature, clinically significant complications associated with cement are exceedingly rare.[12]
Surgical open reduction internal fixation
Surgical fixation of SIFs is usually reserved when other treatment options have failed, the fracture is displaced more than 1 cm, or there is neurological compromise. The three main techniques for surgical fixation of sacral fractures are percutaneous screw fixation, posterior tension band plating, and iliosacral/lumbopelvic fixation.[38] The main disadvantages to surgical fixation are risks associated with general anesthesia, prolonged wound healing times, infection, and potential hardware complication.
CASES SERIES OF PATIENTS TREATED WITH PERCUTANEOUS SACROPLASTY FOR SIF
Four cases of sacroplasty for SIFs were performed at our institution from 2006 to 2016. All cases were performed within the radiology department, two by neuroradiology and two by interventional radiology. All cases of SIF were confirmed through MRI. None of the cases utilized bone scintigraphy. Two cases utilized CT evaluation before sacroplasty. All of the patients were female. The underlying etiology for SIF in three of the cases was osteoporosis and one with an unknown mechanism. Three cases were classified as Denis 3 and one as Denis 1. Three cases utilized CT guidance and one utilized fluoroscopic guidance with post-procedural cone-beam CT to verify cement placement. All of the cases were considered a technical success without evidence of cement leak. All patients stated various degrees of pain reduction, with a majority falling into the categories of complete or near complete reduction in pain levels. Table 2 summarizes the age, gender, etiology, Denis classification, image guidance, approach, and degree of pain reduction following treatment.
| Patients treated with percutaneous sacroplasty for sacral insufficiency fractures | |||||||
|---|---|---|---|---|---|---|---|
| Case | Age (years) | Gender | Etiology | Denis | Image guidance | Approach | Pain Reduction |
| 1 | 94 | Female | Osteoporosis | 3 | CT | Posterior | Near Complete |
| 2 | 65 | Female | Osteoporosis | 1 | CT | Posterior | Complete |
| 3 | 66 | Female | Unknown | 3 | Fluoroscopy and cone-beam CT | Posterior | Near Complete |
| 4 | 74 | Female | Osteoporosis | 3 | CT | Posterior | Partial reduction |
CT: Computed tomography
Case 1
A 94-year-old woman with a history of chronic kidney disease, hypertension, hypothyroidism, and osteoporosis presented with low back pain. MRI of the lumbar spine was first performed and revealed areas of abnormal T2 signal abnormality in the bilateral sacral ala and anterolisthesis of S1 on S2, classifying the fracture as three subtype 2, and is shown in Figure 8a and b. CT of the sacrum was performed for further characterization before sacroplasty and is shown in Figure 8c and d. Bilateral sacroplasty was performed with a posterior approach using only CT guidance, as demonstrated in Figure 8e and f. The procedure was a technical success without evidence of cement leak. The patient described little to no pain immediately post-procedure. Unfortunately, the patient was lost to follow-up and long-term outcome is unknown.

- A 94-year-old woman presenting with low back pain. (a) Axial T2 magnetic resonance (MR) image of the lumbar spine demonstrates abnormal T2 signal in the bilateral sacral wings. (b) Sagittal T2 fat saturation MR image reveals anterolisthesis at S1–2 level and bone marrow edema. (c) Non-contrast coronal computed tomographic (CT) image demonstrates a fracture line in the right sacral ala (arrow) and sclerosis of the left sacral wing (dashed oval). (d) Non-contrast sagittal CT image reveals anterolisthesis of S1 on S2, classifying this SIF as Denis 3, subtype 2. (e) Intraprocedural axial CT image in prone position demonstrates a posterior approach with trocar needle present in the bilateral sacral wings with tips abutting the anterior sacral cortex. (f) Immediate post-procedural axial CT image with the patient in the prone position reveals satisfactory cement placement.
Case 2
A 65-year-old female with a history of osteoporosis presented with acute onset of low back pain when shoveling snow. MRI of the lumbar spine was first performed which revealed no acute lumbar spine abnormality; however, there was partially visualized marrow edema within the right sacral wing, as shown in Figure 9a. Dedicated sacral MRI of the sacrum was then performed and revealed extensive edema within the right sacral ala, consistent with SIF, and shown in Figure 9b. Conservative management was first attempted, but the patient developed new left-sided low back pain. Repeat sacral MRI was performed and revealed new edema in the left sacral wing and persistent right sacral wing edema, as shown in Figure 9c. This fracture is classified as Denis 1. The patient was then referred to interventional radiology by orthopedic surgery. Bilateral sacroplasty was performed with a posterior approach utilizing only CT guidance, as shown in Figure 9d-f. The procedure was a technical success without evidence of cement leak. On 2-week clinic follow-up, the patient had complete resolution of pain.

- A 65-year-old female presented with acute onset of low back pain when shoveling snow. (a) Sagittal T2 magnetic resonance (MR) image through the right sacral ala demonstrates bone marrow edema suggestive of sacral insufficiency fracture (SIF) but not fully evaluated on this dedicated lumbar spine MRI. (b) Axial T2 fat suppressed MR image on dedicated sacral imaging reveals bone marrow edema in the right sacral consistent with Denis 1. (c) Axial T2 fat suppressed MR image performed after conservative therapy failed and the patient developed contralateral pain reveals new area of edema in the left sacral ala and persistent right sacral wing edema. (d) Intraprocedural axial computed tomographic (CT) image with the patient in the prone position demonstrates posterior approach with trocar needles present in the bilateral sacral ala. (e and f) Immediate post-procedural axial CT images reveal satisfactory cement distribution bilaterally without leak.
Case 3
A 66-year-old female with a history of GERD, hyperlipidemia, sleep apnea, hypertension, fibromyalgia, and arthritis initially presented to pain clinic for exacerbation of her low back pain with radiation into the lower extremities for 2 months. The pain was worse with physical activity and better with analgesic medications and rest. No bowel or bladder symptoms. The patient states that she has had exacerbations in the past but none as severe as this current episode, requiring her to use crutches for ambulation. She denied any trauma or inciting event. On physical examination, she had exquisite tenderness to palpation of the bilateral posterior superior iliac spines. MRI of the lumbar spine was performed and revealed edema in both sacral ala and kyphotic angulation, consistent with Denis type 3, and is shown in Figure 10a-c. She was referred to interventional radiology, where she was deemed an appropriate candidate for sacroplastly.

- A 66-year-old female presenting with acute on chronic lower back pain with radiation into the lower extremities. Sagittal short tau inversion recovery (STIR) magnetic resonance (MR) image of the sacrum demonstrates abnormal T2 signal in the (a) right and (b) left sacral alae. (c) Sagittal STIR MR image of the midline sacrum reveals bone marrow edema in the S2 body with mild kyphotic curvature, consistent with Denis 3, subtype 1 fracture. (d) Intraprocedural lateral fluoroscopic image of the sacrum with posterior approach demonstrates trocar needles present in the S1 and S2 sacral wings bilaterally. (e) Anterior oblique intraprocedural fluoroscopic image of the left sacrum with bilateral S1 and S2 needles in place. (f) Immediate post-procedural axial cone-beam computed tomographic (CT) image with (g) coronal reformat reveal appropriate position of the cement within the bilateral sacral wings without leak.
Sacroplasty was performed with a posterior approach using fluoroscopy, as shown in Figure 10d and e. Post-procedural cone beam was performed to confirm appropriate cement distribution, as shown in Figure 10f and g. The procedure was a technical successful without complication. The patient was then seen 1 month later in clinic and stated a one-point reduction in pain. At 3-month clinic follow-up, the patient stated a near complete resolution of pain.
Case 4
A 74-year-old woman with a history of osteoporosis and L1 compression fracture status post-kyphoplasty presented with acute on chronic severe low back pain with radiation into both hips after a fall. Pain was described as 10/10 without improvement with multiple tablets of hydromorphone daily. Radiograph of the pelvis was first performed and did not reveal fracture, as shown in Figure 11a. MRI of the sacrum was then performed and revealed T2 signal abnormality throughout the bilateral sacral wings and anterolisthesis of S3 and S4, consistent with Denis 3, subtype 2. Figure 11b and c demonstrates the fracture on axial and sagittal MRI images. CT was performed for further characterization and revealed a cortical break in the superior left sacral wing and displaced fracture of the right superior pubic rami, as shown in Figure 11d and e.

- A 74-year-old woman with a history of L1 compression fracture status post-kyphoplasty presenting with acute on chronic severe low back pain with radiation into both hips after a fall. (a) Initial anterior posterior radiograph of the pelvis did not reveal significant bony abnormality. (b) Axial T2 fat saturation magnetic resonance (MR) image of the sacrum demonstrates diffuse sacral edema (dashed oval). (c) Sagittal T2 fat saturation MR image through midline pelvis reveals anterolisthesis of S3 on S4 level, consistent with Denis 3, subtype 2 fracture. (d) Axial computed tomographic (CT) images of the pelvis demonstrate a cortical break in the left sacral wing. (e) right superior pubic rami fracture. (f, g) Intraprocedural axial CT images in the prone position demonstrate posterior approach trocar needle placement in the bilateral sacral wings. (h) Immediate post-procedural coronal CT through the sacrum reveals adequate cement distribution without leak.
The patient was then referred to interventional radiology for sacroplasty and the patient was deemed an appropriate candidate. Bilateral sacroplasty was performed utilizing only CT guidance with a posterior approach and is demonstrated in Figure 11f-h. The procedure was a technical success without cement leak. 1-week post-procedure, the patient had significant reduction pain, with a 70% reduction in required narcotics. Clinical follow-up at 10 weeks, the patient described further significant improvement in pain.
DISCUSSION
SIFs are most commonly seen in the elderly female population with underlying post-menopausal osteoporosis.[35] The diagnosis of SIFs is often delayed dueto lack of physician awareness and falsely attributing symptoms to spondylosis.[15,39] A thorough physical examination can localize the pain to the sacrum and direct timely and appropriate imaging. Initial imaging should consist of radiographs, followed by MRI for confirmation.[6] Bone scintigraphy can be performed if MRI is unavailable. CT is utilized for fracture characterization if invasive intervention is being considered.[6]
Management of SIFs consists of non-invasive therapy with or without invasive intervention. However, sacroplasty has been shown to provide early pain relief and early ambulation, compared to medical management alone.[8,9,11] In 2017, Frey et al. published a long-term prospective study comparing percutaneous sacroplasty to non-surgical medical management in 241 SIF patients.[10] There was statistically significant decreased pain, decreased opioid use, and increased patient satisfaction in the sacroplasty cohort compared to the medically managed group at 4, 12, 24, and 52 weeks.[10] There was also continued significant satisfaction with the sacroplasty cohort at 10-year follow-up.[10]
The major theoretical risks of sacroplasty are neurological compromise due to cement leak into the foramina or central canal and cement pulmonary embolism, although reports of these complications are rare. In 2008, Frey et al. published a prospective study which observed 52 patients following percutaneous sacroplasty, with one case of transient radiculitis and no reports of cement pulmonary embolism or permanent neurological compromise.[9]
The two main needle approaches utilized for percutaneous sacroplasty consist of posterior and long-axis. In 2006, Binaghi et al. suggested that long access technique may be the optimal approach as it allows a single needle per treatment side, better cement distribution, and decreased risk of anterior cortex violation, compared to posterior approach.[8] However, in a 2009 meta-analysis by Bayley et al., a majority of operators still prefer a posterior approach over a long-axis approach, likely due to operator comfort with a similar technique when performing vertebroplasty.[40] High-quality prospective studies directly comparing posterior approach to long-axis approach would be useful in determining the ideal technique for sacroplasty.
In our experience, percutaneous sacroplasty with a posterior approach performed for SIFs resulted in significant pain reduction and without any significant complications.
CONCLUSION
Sacral insufficiency fracture is an underdiagnosed debilitating condition that clinicians should be aware of in patients presenting with lower back pain. MRI is currently the preferred imaging modality for the confirmation of SIFs. Percutaneous image-guided sacroplasty has proven to be a safe and effective treatment for SIFs and its use is further supported in our own personal experience with the procedure.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Spontaneous osteoporotic fracture of the sacrum. An unrecognized syndrome of the elderly. JAMA. 1982;248:715-7.
- [CrossRef] [PubMed] [Google Scholar]
- Sacral insufficiency fractures: Current concepts of management. Osteoporos Int. 2006;17:1716-25.
- [CrossRef] [PubMed] [Google Scholar]
- Insufficiency fractures of the sacrum. Twenty cases and review of the literature. Spine (Phila Pa 1976). 1993;18:2507-12.
- [CrossRef] [Google Scholar]
- Fractures of the sacrum caused by bone insufficiency. Meta-analysis of 508 cases. Presse Med. 1997;26:1568-73.
- [Google Scholar]
- Sacral insufficiency fractures: An easily overlooked cause of back pain in elderly women. Arch Intern Med. 1996;156:668-74.
- [CrossRef] [PubMed] [Google Scholar]
- ACR appropriateness criteria® stress (fatigue/insufficiency) fracture, including sacrum, excluding other vertebrae. J Am Coll Radiol. 2017;14:S293-306.
- [CrossRef] [PubMed] [Google Scholar]
- Sacroplasty: A new treatment for sacral insufficiency fracture. J Vasc Interv Radiol. 2002;13:1265-7.
- [CrossRef] [Google Scholar]
- A new, easy, fast, and safe method for CT-guided sacroplasty. Eur Radiol. 2006;16:2875-8.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous sacroplasty for osteoporotic sacral insufficiency fractures: A prospective, multicenter, observational pilot study. Spine J. 2008;8:367-73.
- [CrossRef] [PubMed] [Google Scholar]
- Sacroplasty: A ten-year analysis of prospective patients treated with percutaneous sacroplasty: Literature review and technical considerations. Pain Physician. 2017;20:E1063-72.
- [Google Scholar]
- Percutaneous sacroplasty using CT fluoroscopy. AJNR Am J Neuroradiol. 2006;27:356-8.
- [Google Scholar]
- Sacroplasty by CT and fluoroscopic guidance: Is the procedure right for your patient? AJNR Am J Neuroradiol. 2007;28:38-41.
- [Google Scholar]
- Dual-energy x-ray absorptiometry in the diagnosis of osteoporosis: A practical guide. AJR Am J Roentgenol. 2011;196:897-904.
- [CrossRef] [PubMed] [Google Scholar]
- Sacral stress fractures. J Womens Health (Larchmt). 2003;12:879-88.
- [CrossRef] [PubMed] [Google Scholar]
- The management of sacral stress fractures: Current concepts. Clin Cases Miner Bone Metab. 2011;8:19-23.
- [Google Scholar]
- Neurological complications in insufficiency fractures of the sacrum. Three case-reports. Rev Rhum Engl Ed. 1999;66:109-14.
- [Google Scholar]
- MRI and CT of insufficiency fractures of the pelvis and the proximal femur. AJR Am J Roentgenol. 2008;191:995-1001.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous sacroplasty: Long-axis injection technique. AJR Am J Roentgenol. 2006;186:1252-5.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of planar scintigraphy alone and with SPECT for the initial evaluation of femoral neck stress fracture. AJR Am J Roentgenol. 2008;191:1010-5.
- [CrossRef] [PubMed] [Google Scholar]
- Radionuclide bone imaging: An illustrative review. Radiographics. 2003;23:341-58.
- [CrossRef] [PubMed] [Google Scholar]
- Sacral fractures: An important problem. Retrospective analysis of 236 cases. Clin Orthop Relat Res. 1988;227:67-81.
- [CrossRef] [Google Scholar]
- Biomechanical considerations of fracture treatment and bone quality maintenance in elderly patients and patients with osteoporosis. Clin Orthop Relat Res. 2004;425:12-25.
- [CrossRef] [Google Scholar]
- Cyclo-oxygenase 2 function is essential for bone fracture healing. J Bone Miner Res. 2002;17:963-76.
- [CrossRef] [PubMed] [Google Scholar]
- Recent opioid use and fall-related injury among older patients with trauma. CMAJ. 2018;190:E500-6.
- [CrossRef] [PubMed] [Google Scholar]
- Bisphosphonates: Effects on osteoblast. Eur J Clin Pharmacol. 2012;68:1013-8.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term safety of bisphosphonates. J Clin Endocrinol Metab. 2005;90:1897-9.
- [CrossRef] [PubMed] [Google Scholar]
- Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance. Proc Natl Acad Sci U S A. 2017;114:8722-7.
- [CrossRef] [PubMed] [Google Scholar]
- The effect of teriparatide on fracture healing of osteoporotic patients: A meta-analysis of randomized controlled trials. Biomed Res Int. 2016;2016:6040379.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of teriparatide on unstable pertrochanteric fractures. Biomed Res Int. 2015;2015:568390.
- [CrossRef] [PubMed] [Google Scholar]
- PTH 1-34 (teriparatide) may not improve healing in proximal humerus fractures. A randomized, controlled study of 40 patients. Acta Orthop. 2016;87:79-82.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanism of action of strontium ranelate: What are the facts? Clin Cases Miner Bone Metab. 2010;7:17-8.
- [Google Scholar]
- Percutaneous sacroplasty for the treatment of sacral insufficiency fractures. AJR Am J Roentgenol. 2005;184:1956-9.
- [CrossRef] [PubMed] [Google Scholar]
- PMMA cementoplasty in symptomatic metastatic lesions of the S1 vertebral body. Cardiovasc Intervent Radiol. 2000;23:235-7.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging and treatment of sacral insufficiency fractures. AJNR Am J Neuroradiol. 2010;31:201-10.
- [CrossRef] [PubMed] [Google Scholar]
- Sacral vertebral augmentation: Confirmation of fluoroscopic landmarks by open dissection. Pain Physician. 2008;11:57-65.
- [Google Scholar]
- An easily identifiable anatomic landmark for fluoroscopically guided sacroplasty: Anatomic description and validation with treatment in 13 patients. AJNR Am J Neuroradiol. 2009;30:1070-3.
- [CrossRef] [PubMed] [Google Scholar]
- Open reduction internal fixation of displaced sacral fractures: Technique and results. Orthopedics. 2010;33:730.
- [CrossRef] [PubMed] [Google Scholar]
- Sacral insufficiency fracture, usually overlooked cause of lumbosacral pain. J Korean Neurosurg Soc. 2008;44:166-9.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical outcomes of sacroplasty in sacral insufficiency fractures: A review of the literature. Eur Spine J. 2009;18:1266-71.
- [CrossRef] [PubMed] [Google Scholar]






