Translate this page into:
Ten-year Experience with Percutaneous Cholecystostomy for Acute Cholecystitis in Men

Corresponding Author: Melissa F. Perkal, Department of Surgery, VA Connecticut Healthcare System, 950 Campbell Avenue, West Haven, CT 06516, USA. Phone: +1-203-932-5711, Ext. 4433. E-mail: Melissa.Perkal@va.gov
-
Received: ,
Accepted: ,
How to cite this article: Grisotti G, King JT, Bhattacharya B, Schlessel RB, Gunabushanam G, Perkal MF. Ten-year Experience with Percutaneous Cholecystostomy for Acute Cholecystitis in Men. Am J Interv Radiol 2017, 1(6) 1-6.
Abstract
Objective:
To retrospectively analyze the treatment outcomes of male patients who underwent emergent percutaneous cholecystostomy (PC) for biliary decompression in acute cholecystitis.
Methods:
A single-institution retrospective analysis of 132 patients from 2003 to 2013. Outcome measures were survival, cholecystostomy drain outcomes, and definitive treatment with surgical cholecystectomy.
Results:
The patient population was all male, with a mean age of 70.6 years. 79 patients (59.9%) were admitted for biliary disease and 34 patients (25.8%) were in an intensive care unit (ICU) when diagnosed. 18 patients (13.6%) died within 30 days of PC, an additional 12 (9%) died within 6 months of PC, and the median survival was 4.9 years. Multivariate logistic regression showed a direct relationship between 1 month and 6-month mortality with total bilirubin and an inverse relationship with hematocrit. Cox regression analysis for long-term survival revealed increased mortality associated with respiratory failure (hazard ratio [HR]: 2.40, P = 0.023) and total bilirubin (HR: 1.11, P < 0.001); lower mortality was associated with a primary diagnosis of cholecystitis (HR: 0.29, P = 0.010), higher hematocrit (HR: 0.89, P < 0.001), and ICU (HR: 0.17, P = 0.003) and floor (HR: 0.34, P = 0.029) diagnosis location. Only 55 patients (41.7%) proceeded to surgical cholecystectomy. Outcomes at 1 year (n = 110) were 40% alive after surgery, 25.5% alive without surgery or a drain, 2.7% alive without surgery but with a drain, and 31.8% dead.
Conclusions:
Although PC is associated with a high early mortality, 25.5 % of our patients were definitively treated with cholecystostomy alone. Certain biomarkers and patient characteristics may help model survival after PC.
Keywords
Acute cholecystitis
gallbladder inflammation
percutaneous cholecystostomy
INTRODUCTION
Surgical cholecystostomy was first described in the late 19th century. Nearly a century later, a minimally invasive percutaneous cholecystostomy (PC) was first performed in 1979 for obstructive jaundice,[1] and then, in 1980 for acute cholecystitis.[2] PC became a recognized temporizing measure for patients with cholecystitis, either with or without cholelithiasis, who were too debilitated or acutely ill to undergo an emergent surgical cholecystectomy, for which rates of morbidity up to 41% and perioperative mortality up to 18% have been reported.[3] Recently, PC has also been proposed as a preferred therapeutic intervention, sometimes definitively, in pregnant patients[4] and in chronic hemodialysis patients.[5] However, a Cochrane Review from 2013 found that more information is still needed to determine the role of PC in high-risk surgical patients.[6] Cholecystostomy may be of utility not only in high-risk patients, categorized as such due to their own comorbidities, but also in high-risk cholecystitis, in relatively healthier patients for whom cholecystectomy may have an increased risk of complications or conversion from laparoscopic to open surgery due to the extent of gallbladder inflammation or edema.[7] It has also been postulated that cholecystostomy may serve as definitive treatment for cholecystitis, without a need for interval cholecystectomy.[8,9] The indications for cholecystostomy and for interval cholecystectomy are important clinical questions as the extent of biliary disease is increasing. Gallstones affect 10–15% of the adult population, meaning 20–25 million Americans have gallstones, and this number will only continue to rise with the increase in obesity and metabolic syndrome.[10] We retrospectively studied all patients that underwent PC at a single institution to evaluate predictors of outcomes and the fate of the cholecystostomy tube (either interval surgical cholecystectomy or tube removal).
MATERIALS AND METHODS
This retrospective study was approved by the institutional review board, and the requirement for informed consent from individual patients was waived. We used an interventional radiology quality assurance database and the electronic medical record to retrospectively identify and collect data on all patients undergoing PC at our single institution from November 2003 to November 2013. Data collection included demographics (age, gender), primary diagnosis, cholecystitis diagnosis location (emergency department (ED), intensive care unit (ICU), and floor), body temperature, laboratory values (blood hematocrit and white blood cell count, serum albumin, total bilirubin, and serum alkaline phosphatase), imaging findings (gallbladder wall thickening, stones or sludge on the right upper quadrant ultrasound, or computed tomography scan), comorbid conditions (respiratory failure) (defined as requiring mechanical ventilation), renal failure (defined as requiring dialysis), and sepsis (either diagnosed in the chart by an attending or patient noted to have at least two of the following: Abnormal temperature, leukocytosis, or tachycardia), procedure dates (PC and open or laparoscopic cholecystectomy), American Society of Anesthesiologists physical status classification system (ASA), cholecystostomy tube removal interval and complications, discharge disposition (home, hospice, extended care facility, transfer, and dead), date of death, cause of death (sepsis, stroke, cardiac arrest, cancer, respiratory failure, other, and unknown), and date of last follow-up. In the univariate and bivariate analyses, age was grouped in decades, ASA combined into 1–3 or 4–5, and procedure year was dichotomized on the median at 2003–2009 and 2010–2013. Temperature and laboratory values were expressed as means and standard deviations. We used our Surgical Quality Improvement Program case log to identify the number of surgical cholecystectomies done during the same time period.
We used logistic regression to study all-cause mortality at 30, 180, and 365 days after PC. For each survival interval, simple logistic regression was used to screen individual variables, and all variables with P < 0.10 in the simple logistic regressions were entered into a stepwise logistic regression to generate the final multivariate logistic regression model. We also used Kaplan–Meier plots, Cox regression, and an analogous sequential simple and multivariate regression model building technique to examine long-term survival following PC.
Some patients underwent open or laparoscopic cholecystectomy following PC. Kaplan–Meier plots were used to display the intervals between cholecystostomy and cholecystectomy. We used simple and multivariate competing-risks regressions to study factors associated with undergoing surgery after cholecystostomy while accounting for differential survival following the initial procedure.
Analyses were performed using Stata version 13.1 (StataCorp, College Station, TX). P <0.05 was considered significant, and those <0.10 and >0.05 were considered trends.
RESULTS
Patient characteristics
From November 2003 to November 2013, 434 surgical cholecystectomies were performed at our institution. During this same time period, a total of 132 patients underwent PC (Figure 1). All of the patients were male, and the mean age was 70.6 years with a quarter of the patients over the age of 80 years (Table 1). The health-care facility’s bed capacity remained the same in this timeframe. Of the patients who eventually received a PC, half were diagnosed on presentation to the ER, while the diagnosis location for the remaining patients was split between the general hospital ward and the ICU. At the time of diagnosis, 24.2% of patients had respiratory failure, 18.9% of patients had renal failure, and 37.1% of patients were septic. On imaging, 26.7% of patients had neither gallstones nor sludge and were assumed to have acalculous cholecystitis.
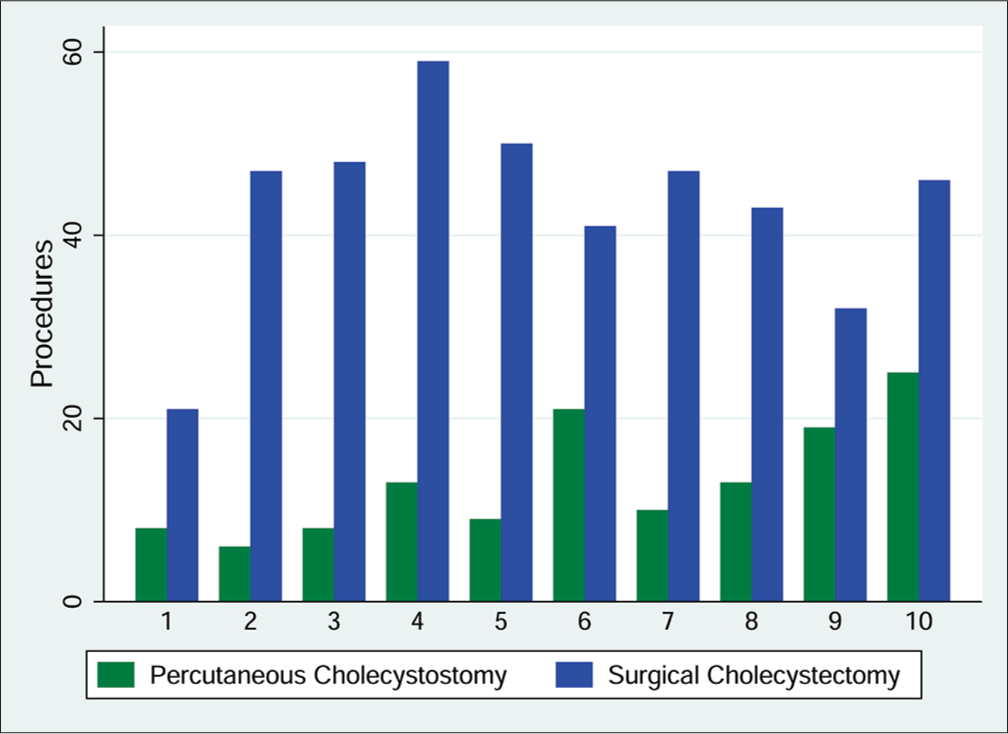
- Comparison of number of percutaneous cholecystostomies and surgical cholecystectomies done per study year.
| Variable | Value | |
|---|---|---|
| Age | ||
| <60 years | 23 (17.4%) | |
| 60–69 years | 47 (35.6%) | |
| 70–79 years | 29 (21.9%) | |
| >80 years | 33 (25%) | |
| Gender | ||
| Male | 132 (100%) | |
| Comorbidities | ||
| Respiratory failure | 32 (24.2%) | |
| Renal failure | 25 (18.9%) | |
| Sepsis | 49 (37.1%) | |
| Percutaneous cholecystostomy procedure year | ||
| 2003-2009 | 66 (50%) | |
| 2010-2013 | 66 (50%) | |
| Cholecystitis diagnosis location | ||
| ED | 69 (52.3%) | |
| ICU | 34 (25.8%) | |
| Floor | 29 (22%) | |
| Cholecystitis is primary diagnosis | 79 (59.9%) | |
| Imaging findings | ||
| Wall thickening | 77 (58.8%) | |
| Stones or sludge | 96 (73.3%) | |
| Temperature (° Fahrenheit), mean (SD) | 99.2 (1.66) | |
| Hematocrit (%), mean (SD) | 34.5 (6.2) | |
| WBC (×109/L), mean (SD) | 15.29 (8.30) | |
| Albumin (g/dL), mean (SD) | 2.85 (0.88) | |
| Total bilirubin (mg/dL), mean (SD) | 2.59 (3.39) | |
| Alkaline phosphatase (U/L), mean (SD) | 192.98 (190.19) | |
| ASA score 2003–2009 | ||
| 1-3 | 51 (77.3) | |
| 4-5 | 15 (22.7) | |
| ASA score 2010-2013 | ||
| 1-3 | 65 (98.5%) | |
| 4-5 | 1 (1.5%) | |
ASA: American Society of Anesthesiologists’ physical status classification, ED: Emergency department, ICU: Intensive care unit, SD: Standard deviation, WBC: White blood cell count unit, SD: Standard deviation, WBC: White blood cell count
Survival
The procedure itself was technically successful (defined as confirmation of catheter placement within the lumen of the gallbladder with imaging)[11] with only one periprocedure death (associated with severe preprocedure cardiopulmonary disease) and one patient was taken emergently to the operating room within 24 h of procedure for concern of procedure-associated bowel perforation. On exploration in the operating room, there was no spillage of enteric contents.
Eighteen patients (13.6%) died within 30 days of PC. The most common cause of death was sepsis (n = 9, 50%), followed by cancer (n = 3, 20%), and respiratory failure (n = 2, 13.3%). Only one additional patient died within the next 30 days from sepsis. 12 patients died between 31 and 180 days after PC, for a cumulative 180-day mortality rate of 22.7%. The most common causes of death were cancer (n = 4, 33.3%), sepsis (n = 3, 25%), respiratory failure (n = 2, 16.7%), and other (n = 2, 16.7%). Multivariate logistic regression analysis (Table 2) showed a significant relationship between 30- and 180-day mortality with a lower hematocrit and higher total bilirubin.
| Variable | OR | 95% CI | p value |
|---|---|---|---|
| 30-day mortality | |||
| Hematocrit | 0.74 | 0.64, 0.86 | <0.001 |
| Total bilirubin | 1.21 | 1.05, 1.41 | 0.009 |
| 180-day mortality | |||
| Hematocrit | 0.79 | 0.71, 0.87 | <0.001 |
| Total bilirubin | 1.15 | 1.01, 1.30 | 0.030 |
| 1-year mortality | |||
| Hematocrit | 0.77 | 0.69, 0.86 | <0.001 |
OR: Odds ratio, CI: Confidence interval
Between 180 and 365 days after PC, an additional five patients died for an aggregate 365-day mortality rate of 26.5%. The deaths were caused by cancer (n = 2, 40%), sepsis (n = 1, 20%), stroke (n = 1, 20%), and other (n = 1, 20%). Multivariate logistic regression (Table 2) showed a relationship between 1-year mortality and lower hematocrit (odds ratio: 0.77, 95% confidence interval: 0.69, 0.86; P < 0.001).
The study cohort experienced 61 deaths and 322.5 person-years of follow-up, and median survival after PC was 4.9 years. The most common causes of death were cancer (n = 18, 29.5%), sepsis (n = 18, 29.5%), respiratory failure (n = 8, 13.1%), and other (n = 8, 13.1%). A Kaplan-Meier plot (Figure 2) illustrated the relatively high rate of mortality within the first 3 months after PC, followed by a survival plateau for several years, and then, an increase in mortality rate after 6 years. In the multivariate Cox regression model (Table 3), increased mortality was associated with respiratory failure (hazard ratio (HR): 2.40, P = 0.023) and total bilirubin (HR: 1.11, P = 0.001); lower mortality was associated with a primary diagnosis of cholecystitis (HR: 0.29, P = 0.010), higher hematocrit (HR: 0.89, P < 0.001), and a non-ED cholecystitis diagnosis in the ICU (HR: 0.17, P = 0.003) or on the floor (HR: 0.34, P = 0.029). The median length of stay after PC was 7.5 days (interquartile range from 4 to 20 days) with 55% of patients being discharged home.
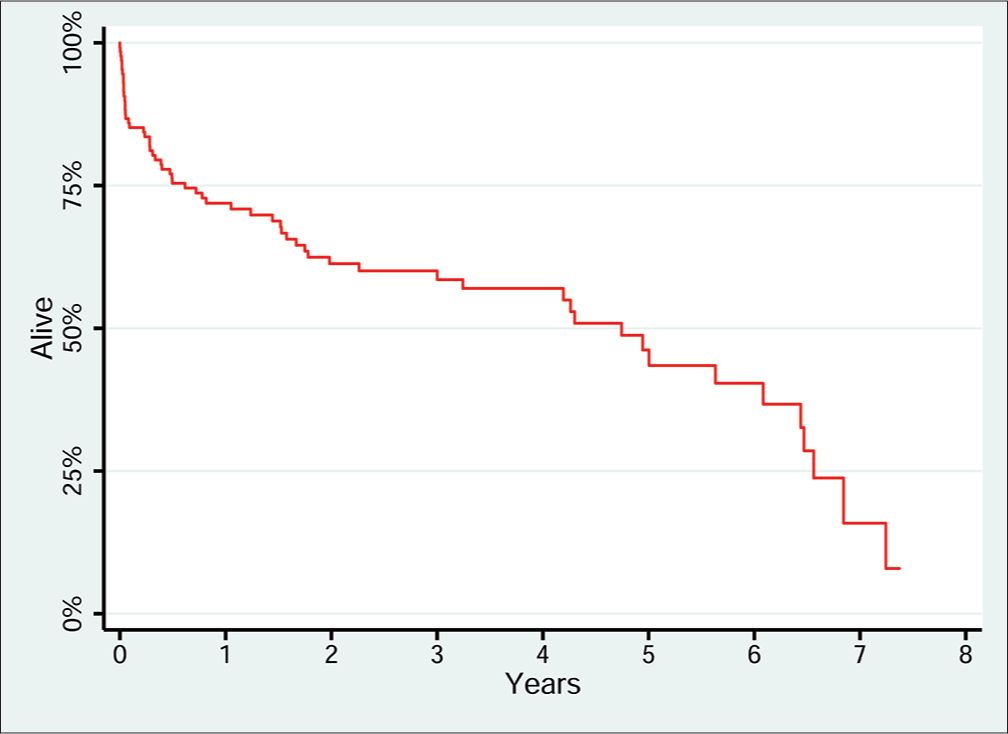
- Long-term survival after percutaneous cholecystostomy. Kaplan-Meier plot of long term survival after percutaneous cholecystostomy, n=132. Median survival after percutaneous cholecystostomy is 4.9 years.
| Variable | Hazard ratio | 95% CI | p value |
|---|---|---|---|
| Hematocrit | 0.89 | 0.84, 0.95 | <0.001 |
| Total bilirubin | 1.11 | 1.06, 1.18 | <0.001 |
| Primary diagnosis of cholecystitis | 0.29 | 0.11, 0.74 | 0.010 |
| Diagnosis location | |||
| ED | 1.00 | - | - |
| ICU | 0.17 | 0.05, 0.54 | 0.003 |
| Floor | 0.34 | 0.13, 0.89 | 0.029 |
| Respiratory failure | 2.40 | 1.13, 5.11 | 0.023 |
CI: Confidence interval, ED: Emergency department, ICU: Intensive care unit, PC: Percutaneous cholecystostomy
Surgical cholecystectomy
Fifty-five patients (41.7%) underwent cholecystectomy after PC (Table 4). Of the patients who eventually underwent surgical cholecystectomy, the majority of procedures occurred at the 6–12 weeks time period after the PC (Figure 3). While 56 drains were removed preoperatively or intraoperatively at the time of cholecystectomy, 30% of patients had the drain removed after either clamping trials or cholangiography demonstrated resolution of acute cystic duct obstruction. At the time of data collection for this study, three patients still had the drain in place, while a quarter of patients died with the drain in place. Surgical cholecystectomy was performed laparoscopically in 36 patients (65.5%) with conversion to open cholecystectomy in 19 patients (34.5%). The median length of stay was 3 days, with 91% of patients discharged to home and 9% to a rehabilitation facility.
| Variable | value | |
|---|---|---|
| Fate of drain | ||
| Removed preoperatively or intraoperatively | 56 (42.4%) | |
| Removed with resolution of cystic duct obstruction | 41 (31.1%) | |
| Drain still in place, patient alive | 3 (2.3%) | |
| Drain in place at time of death | 32 (24.2%) | |
| Time from cholecystostomy to surgery, in weeks | ||
| <6 | 5 (8.9%) | |
| 6-12 | 33 (58.9%) | |
| >12 | 18 (32.1%) | |
| Surgical cholecystectomy | ||
| Laparoscopic | 36 (64.3%) | |
| Laparoscopic with conversion to open | 19 (33.9%) | |
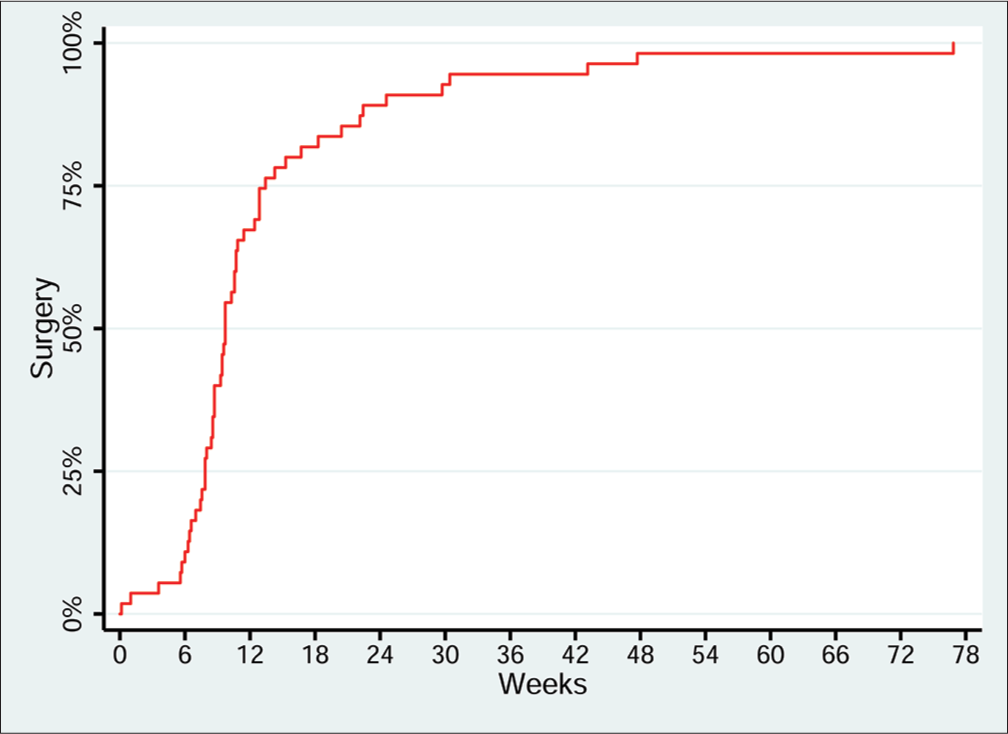
- Lag time between percutaneous cholecystostomy and surgical cholecystectomy. Kaplan-Meier plot of time between percutaneous cholecystostomy and surgicalcholecystectomy, n = 55. Y axis demonstrates cumulative percentage of patients who have undergone surgical cholecystectomy. X axis demonstrates time in weeks from percutaneous cholecystostomy placement to surgery. Median time between percutaneous cholecystostomy and surgery is 68 days.
When considering a 1-year follow-up, 110 patients were available to be evaluated. Of these, 40% were alive after surgery, 25.5% were alive without surgery or a drain, 2.7% were alive without surgery but with a drain, and 31.8% were dead (Figure 4).
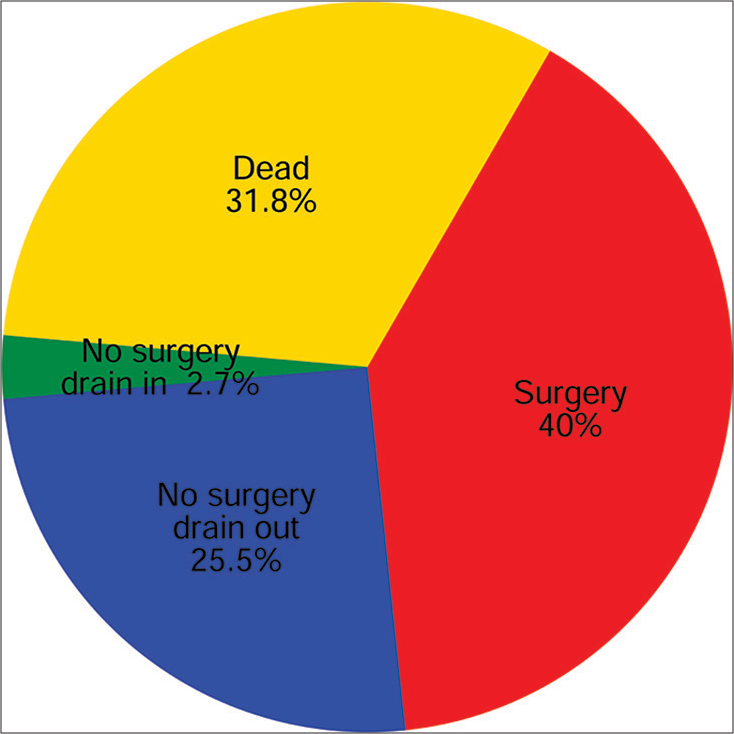
- One-year outcome following percutaneous cholecystostomy. Follow-up data was available for 110 of 132 patients.
DISCUSSION
PC has emerged as an alternative to surgical cholecystectomy for acute calculous and acalculous cholecystitis, providing both diagnostic information about bacterial culture data[12] and therapeutic decompression of the gallbladder for those too ill for a surgical cholecystectomy.[13]
A 12-year retrospective analysis of the US Nationwide Inpatient Sample database, comparing PC and cholecystectomy for patients with either calculous or acalculous cholecystitis, confirmed that patients receiving PC were older with more comorbidities.[14] It was also noted that patients who underwent PC had longer lengths of stay and higher rates of mortality as compared to patients who went directly to surgery, even after adjusting for age, comorbidity, and year. This argues for the need for more definitive criteria of which patient population should be treated with PC. Another long-term retrospective analysis, over 10 years[15] had a higher rate of elective cholecystectomy after PC (64%), including some patients with acalculous cholecystitis.
Compared to these two studies, our study demonstrates that for a significant number of patients, PC becomes definitive therapy for this disease, obviating the need for further surgical intervention in a relatively high-risk population. Our study also brings up the intriguing idea of using PC in healthier patients, where delayed cholecystectomy may be advantageous.
We found an increased trend of utilizing PC in more recent years as a temporizing measure, even in patients with fewer comorbidities. The fact that our patients may have been healthier in the latter half of the study is suggested by comparing the trend of ASA scores. The healthier patients who might be considered for PC include those who may have had symptoms for more than 3 days, suggesting a significant amount of inflammation around the gallbladder;[16,17] thus making early cholecystectomy more prone to complications.
After PC, 42% of patients (55 of 132) proceeded to an elective cholecystectomy. However, 40 patients had removal of the drain with resolution of symptoms and without recurrence at the time of study conclusion. A large part of this group is likely the acalculous cholecystitis cohort, for whom PC may be definitive treatment.[9] In our study, 9% of cholecystectomies were done within the first 6 weeks after PC, but most (59%) were done between 6 and 12 weeks, which coincides with the majority of recommendations in the literature,[18-21] and 32% were done after 12 weeks. More than half of the cholecystectomies (64%) were done laparoscopically, while 34% required conversion to open, coinciding with published rates of conversion of 6–35%.[9,18-19] While skill and ease of laparoscopy have certainly increased, it may be that waiting for the acute inflammation to resolve allows for a lower risk of operative complications.
A third 10-year study revealed that outcomes for PC were better when done when the disease is primary and not precipitated by concurrent illness.[22] Our study is in agreement, with 60% having a diagnosis of primary cholecystitis. Half of the cases were diagnosed on presentation to the ED, while the cases diagnosed as inpatient showed a slight predilection for ICU status as compared to general medical or surgical ward (26% compared to 22%). This correlates to the rate of acalculous cholecystitis of 27% in this study population, as acalculous cholecystitis is usually found in the ICU and fueled by biliary stasis in the absence of enteral nutrition. Median survival was 4.9 years, and at the conclusion of this study, 46% of patients had died, with the top three causes being 18% due to sepsis, 18% due to metastatic cancer, and 8% due to respiratory failure. Only 14% of PC patients died within 30 days of the procedure, again with sepsis as the leading cause. This high rate of mortality due to sepsis can partially be attributed to physicians reaching for PC as a treatment of last resort for a presumed cause of sepsis in an already clinically deteriorating patient.
The limitations of this study include that it is an all-male patient population, considering that biliary disease is found predominantly in women. The study is also limited by it being a single institution study with only retrospective data.
In conclusion, this study validates the recent trend toward performing PC as the definitive treatment, without progression to a delayed cholecystectomy, although there is still no consensus.[23] In addition, this 10-year retrospective analysis may be used in counseling high-risk patients and their families at the time of diagnosis of cholecystitis. Factors, including the presence of respiratory failure and using elevated total bilirubin as a biochemical marker of clinical status, may indicate an increased likelihood of worse outcome.
ACKNOWLEDGMENTS
The abstract was presented at the New England Surgical Society Meeting, September 2014, Stowe, VT, USA, as a poster presentation.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Percutaneous cholecystostomy and cholangiography in patients with obstructive jaundice. Radiology. 1979;130:601-2.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous cholecystostomy. AJR Am J Roentgenol. 1982;139:1240-1.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome after percutaneous cholecystostomy for acute cholecystitis: A single-center experience. J Gastrointest Surg. 2012;16:1860-8.
- [CrossRef] [PubMed] [Google Scholar]
- Minimally invasive management of acute biliary tract disease during pregnancy. HPB Surg. 2009;2009:829020.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous cholecystostomy as a first-line therapy in chronic hemodialysis patients with acute cholecystitis with midterm follow-up. Cardiovasc Intervent Radiol. 2011;34:362-8.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous cholecystostomy for high-risk surgical patients with acute calculous cholecystitis. Cochrane Database Syst Rev. 2013;12:CD007088.
- [CrossRef] [Google Scholar]
- Selective use of tube cholecystostomy with interval laparoscopic cholecystectomy in acute cholecystitis. Arch Surg. 2000;135:341-6.
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology of gallbladder disease: Cholelithiasis and cancer. Gut Liver. 2012;6:172-87.
- [CrossRef] [PubMed] [Google Scholar]
- Emergency cholecystectomy vs percutaneous cholecystostomy plus delayed cholecystectomy for patients with acute cholecystitis. Hepatobiliary Pancreat Dis Int. 2014;13:316-22.
- [CrossRef] [Google Scholar]
- Systematic review of cholecystostomy as a treatment option in acute cholecystitis. HPB (Oxford). 2009;11:183-93.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous cholecystostomy: The radiologist’s role in treating acute cholecystitis. Clin Radiol. 2013;68:654-60.
- [CrossRef] [PubMed] [Google Scholar]
- US-guided percutaneous cholecystostomy: Features predicting culture-positive bile and clinical outcome. Radiology. 2004;230:785-91.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous cholecystostomy in patients with acute cholecystitis: Experience of 45 patients at a US referral center. J Am Coll Surg. 2003;197:206-11.
- [CrossRef] [Google Scholar]
- A nationwide examination of outcomes of percutaneous cholecystostomy compared with cholecystectomy for acute cholecystitis, 1998-2010. Surg Endosc. 2013;27:3406-11.
- [CrossRef] [PubMed] [Google Scholar]
- Revisiting percutaneous cholecystostomy for acute cholecystitis based on a 10-year experience. Arch Surg. 2012;147:416-22.
- [CrossRef] [PubMed] [Google Scholar]
- Can percutaneous cholecystostomy be a definitive management for acute acalculous cholecystitis? J Clin Gastroenterol. 2012;46:216-9.
- [CrossRef] [PubMed] [Google Scholar]
- Emerging indications for percutaneous cholecystostomy for the management of acute cholecystitis-a retrospective review. Int J Surg. 2011;9:456-9.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of early and delayed laparoscopic cholecystectomy for acute cholecystitis: Experience from a single center. N Am J Med Sci. 2013;5:414-8.
- [CrossRef] [PubMed] [Google Scholar]
- Early versus delayed laparoscopic cholecystectomy for acute cholecystitis: A prospective randomized trial. Surg Endosc. 2004;18:1323-7.
- [CrossRef] [PubMed] [Google Scholar]
- Prospective evaluation of patients with acute cholecystitis treated with percutaneous cholecystostomy and interval laparoscopic cholecystectomy. Int J Surg. 2006;4:101-5.
- [CrossRef] [PubMed] [Google Scholar]
- Technique and indications of percutaneous cholecystostomy in the management of cholecystitis in 2014. J Visc Surg.. 2014;151:435-9.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous cholecystostomy for acute cholecystitis: Ten-year experience. J Vasc Interv Radiol. 2012;23:83-8.
- [CrossRef] [PubMed] [Google Scholar]
- Short-and long-term outcomes following percutaneous cholecystostomy for acute cholecystitis in high-risk patients. Surg Endosc. 2012;26:1343-51.
- [CrossRef] [PubMed] [Google Scholar]






