Translate this page into:
Percutaneous glue embolization as an alternative treatment for visceral pseudoaneurysms

*Corresponding author: Jackson Carl Rossborough, Department of Interventional Radiology, University of Miami Miller School of Medicine, Miami, United States. jcr80@miami.edu
-
Received: ,
Accepted: ,
How to cite this article: Rossborough JC, Patel N, Kumar JG, Thornton LM. Percutaneous glue embolization as an alternative treatment for visceral pseudoaneurysms. Am J Interv Radiol. 2024;8:2. doi:10.25259/AJIR_42_2023
Abstract
Visceral artery pseudoaneurysm is a rare, life-threatening medical condition with a high incidence of rupture and bleeding. At most institutions, endovascular intervention is considered the first-line treatment with surgical repair as a secondary option. Percutaneous glue embolization is emerging as a safe alternative technique for minimally invasive treatment of visceral pseudoaneurysms. This case series aims to promote percutaneous glue embolization as a life-saving measure in cases where an endovascular approach cannot be performed successfully, and patients are not surgical candidates.
Keywords
Case series
Embolization
Percutaneous
Visceral pseudoaneurysm
INTRODUCTION
Pseudoaneurysms, also known as “false aneurysms,” occur from damage to one or more layers of the arterial wall, which leads to a hematoma contained by the products of the coagulation cascade at the origin of the damage.[1] In comparison to a true aneurysm, which has intact layers of the arterial wall, the tenuous containment of pseudoaneurysms presents a much higher risk of recurrent bleeding and thus an emergent medical problem.[2] Visceral artery pseudoaneurysms (VAPAs) are particularly challenging due to their proximity to major organs and complex vasculature, including networks of collateral vessels further complicating treatment options.[3,4] At most institutions, the currently preferred treatment for VAPAs is endovascular intervention,[5] during which the site of the pseudoaneurysm or the supplying artery is embolized with a variety of agents including coils, n-butyl cyanoacrylate (n-BCA) glue, and/or stents.[6] This method is considered favorable to open surgery as studies have demonstrated a comparable success rate as well as lower rates of morbidity.[7] In cases with suboptimal anatomy for endovascular treatment, an open surgical technique with plication or grafting is typically performed.[8]
However, advances in image-guided targeting software for interventional procedures have led to improvements in a percutaneous technique,[9] which utilizes the targeting software to percutaneously advance a needle directly into the pseudoaneurysm sac.[10] This is typically followed by injection of an embolic agent such as n-BCA glue for direct embolization.[11] Several case reports have begun to introduce percutaneous glue embolization as a safe and effective treatment for VAPA.[12,13] This study aims to further promote this viable alternative treatment option through the description of several clinical vignettes utilizing a percutaneous glue embolization technique.
CASE REPORT
Approval for this study was obtained from the Hospital’s Institutional Review Board in compliance with institutional standards. A retrospective review of four patients (2 males and 2 females) with visceral pseudoaneurysms treated by percutaneous glue embolization was performed. The clinical history, procedure details, complications, and outcomes of each patient were collected from the medical records [Table 1]. The procedure was effective in all four cases without any significant complications or recurrence as of the most recent follow-up.
| Case | Age | Gender | Relevant Medical History | Presenting Symptoms | VAPA Location | Complications | Length of Follow-up |
|---|---|---|---|---|---|---|---|
| 1 | 83 | Female | Severe celiac stenosis | Sudden-onset abdominal pain and hypotension | Arc of Bühler | None | 12 months |
| 2 | 42 | Male | Pancreatic ampullary carcinoma s/p Whipple | Hemorrhagic shock | Common hepatic artery | None | 42 months |
| 3 | 27 | Female | Chronic pancreatitis, cirrhosis | Abdominal pain, hematemesis, and melena | Superior Branch of the gastroduodenal artery | None | 6 months |
| 4 | 58 | Male | Chronic pancreatitis s/p distal pancreatectomy | Drop in hemoglobin with hypotension | Branch of the Inferior pancreaticoduodenal artery | The bleeding resolved, but the pancreatic leak led to multi-organ failure | 2 weeks |
VAPA: Visceral artery pseudoaneurysm
Case #1
An 83-year-old female with a medical history of breast cancer, atrial fibrillation, stroke, and hypertension presented to the emergency department (ED) with sudden-onset left abdominal pain accompanied by tachypnea and hypotension. Initial imaging with computed tomography angiography (CTA) revealed significant hemorrhagic ascites with active extravasation from a ruptured vessel or potential pseudoaneurysm near the pancreatic head. In addition, there was severe, flow-limiting celiac stenosis with poststenotic dilation of the celiac trunk [Figure 1a and b]. Following hemodynamic resuscitation, a diagnostic angiogram was performed and confirmed celiac stenosis with two robust collateral pathways, an arc of Bühler, and a hypertrophied pancreaticoduodenal arcade [Figure 1c and d]. A large pseudoaneurysm measuring 7 mm was seen arising from a branch of the arc of Bühler. An initial attempt at endovascular glue embolization was made, which successfully embolized this branch; however, there was persistent filling of the pseudoaneurysm on post-embolization angiograms due to retrograde flow through the well-developed pancreaticoduodenal arcade [Figure 1e and f]. The decision was, then, made to pursue a percutaneous approach, as embolization of the VAPA through the pancreaticoduodenal arcade feeders would have potentially caused non-target embolization of the arcade itself resulting in hepatic and splenic ischemia in this patient with celiac stenosis. Using the previously injected liquid embolic as a target, a 22-gauge Chiba needle (Cook Medical, Bloomington, Indiana, USA) was advanced into the pseudoaneurysm sac under fluoroscopy. The position was confirmed by multiple obliques with the Chiba tip adjacent to the previous glue cast [Figure 2a and b]. Embolization was performed using 1 cc of a 2:1 ratio of lipiodol: TRUFILL™ n-BCA Liquid Embolic System glue (CERENOVUS, Irvine, California, USA) mixture. The embolization endpoint was felt to be when the direct feeding vessels as well as the VAPA were filled. Post-embolization angiograms demonstrated cessation of active hemorrhage with preservation of the pancreaticoduodenal arcade. Shortly after direct percutaneous embolization, the patient stabilized hemodynamically while on the angiography table. Repeat CTA performed at 4 weeks post-procedure revealed no evidence of residual VAPA and the patient denies any further episodes of gastrointestinal (GI) bleeding as of the most recent clinic follow-up 12 months after the procedure [Figure 2c].
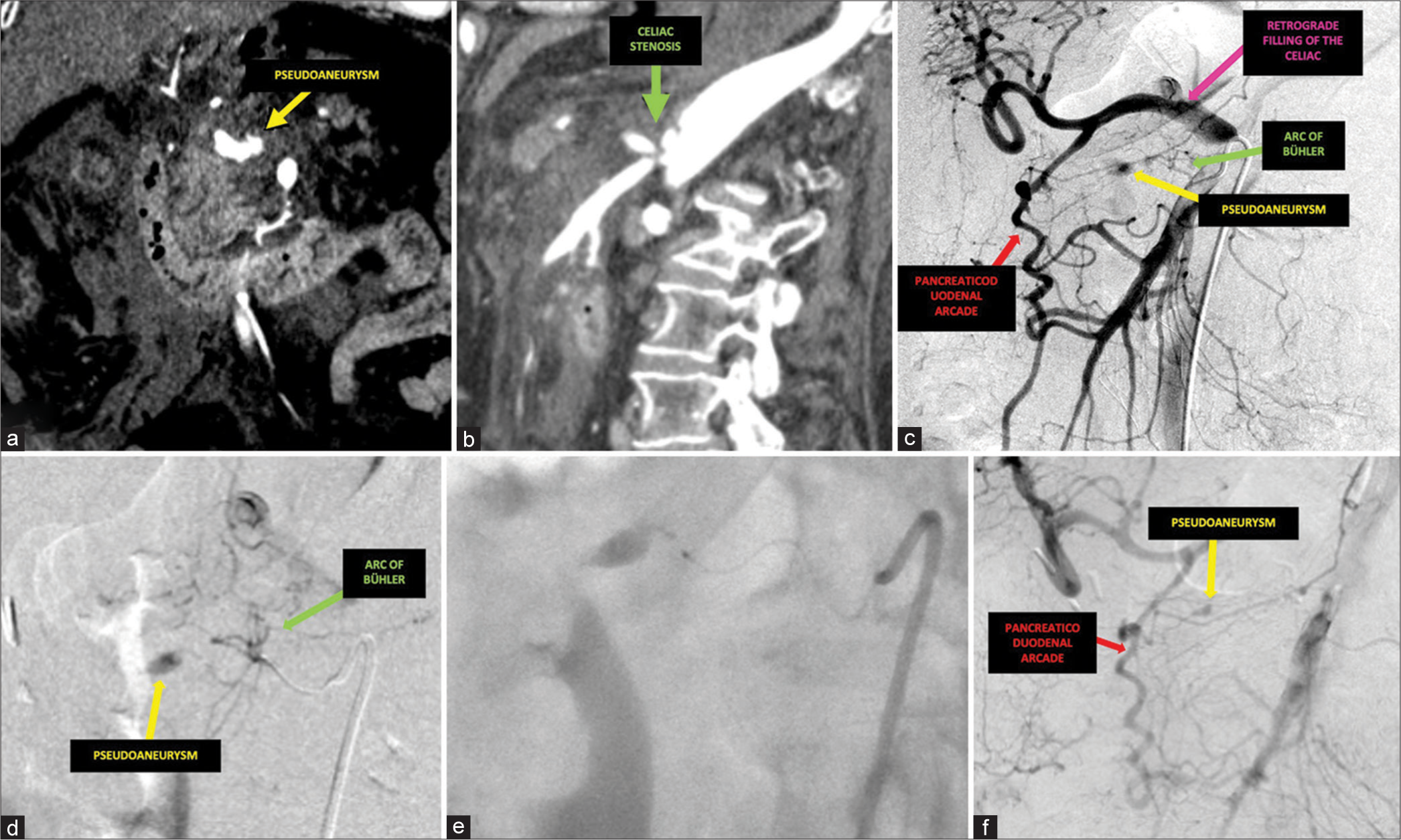
- A 83-year-old female presented with sudden-onset left abdominal pain accompanied by tachypnea and hypotension. (a) Coronal arterial phase computed tomography (CT) demonstrated a visceral pseudoaneurysm (yellow) and (b) sagittal arterial phase CT demonstrated severe celiac stenosis (green) with post-stenotic dilatation. (c) Initial digital subtraction angiogram from the superior mesenteric artery demonstrated a pseudoaneurysm (yellow) fed by a prominent arc of Bühler (green) as well as a hypertrophied pancreaticoduodenal arcade (red) supplying retrograde hepatic and splenic (pink) blood flow. (d) Digital subtraction angiogram after navigation into the branches of the arc of Bühler (green) further confirmed the supply of the pseudoaneurysm (yellow). (e) The microcatheter system was advanced proximal to the pseudoaneurysm and n-butyl cyanoacrylate glue was injected under fluoroscopy. (f) Post-embolization digital subtraction angiogram revealed persistent filling of the pseudoaneurysm (yellow) from the pancreaticoduodenal arcade (red).

- An 83-year-old female presented with sudden onset left abdominal pain accompanied by tachypnea and hypotension. (a) Using the previously injected liquid embolic as a target, a Chiba needle was advanced into the pseudoaneurysm sac under fluoroscopy, and n-butyl cyanoacrylate glue was injected for embolization. (b) Post-embolization digital subtraction angiogram demonstrated no further filling of the pseudoaneurysm. (c) Follow-up at 1 month with coronal 90 s delay phase computed tomography (CT) revealed an appropriate glue cast without evidence of further pseudoaneurysm or extravasation.
Case #2
A 42-year-old male with newly diagnosed pancreatic ampullary carcinoma underwent staging laparotomy and Whipple pancreaticoduodenectomy complicated by postoperative hemorrhage. The patient had a severe episode of hematemesis and hematochezia resulting in hemorrhagic shock on postoperative day 24. CTA revealed a 1.1 cm pseudoaneurysm arising from peripancreatic collaterals arising from the common hepatic artery (CHA) and anastomosing to the splenic artery. An angiogram was performed and confirmed these findings; however, despite multiple attempts, this VAPA was unable to be catheterized anterograde through the CHA [Figure 3a and b]. During this procedure, there was also concern for perforation of a splenic collateral which contributed to the pancreatic arterial arcade; this vessel was coiled given concern for perforation and to decrease flow to the pseudoaneurysm (PSA) [Figure 3c and d]. Given that the patient remained in hemorrhagic shock after the procedure, a multi-disciplinary decision was made to perform a second angiogram and place overlapping stent grafts to exclude small branches from the CHA and gastroduodenal artery (GDA) stump. Despite this effort, a completion angiogram demonstrated a type II endoleak into the pseudoaneurysm sac and post-angiography CTA demonstrated interval rupture of the pseudoaneurysm with active extravasation [Figure 3e and f]. At this point, the anterograde route to the aneurysm was covered with stent grafts and the coils in the splenic collateral prevented retrograde catheterization. Given the patient’s continued critical condition and no alternate surgical option due to concern for “frozen abdomen,” the decision was made to perform percutaneous embolization. Ultrasound could not be used due to shadowing from the abdominal wound vac; therefore, the existing stent was used as a fluoroscopic landmark. The VAPA was accessed with a 25-gauge Chiba needle under fluoroscopy and the position was confirmed with cone-beam computed tomography (CBCT) [Figure 4a and b]. Embolization was performed using 2 cc of a 2:1 ratio of lipiodol: n-BCA glue mixture, injected directly into the pseudoaneurysm sac. The embolization endpoint similarly was reached after allowing for the reflux of the glue into the feeding vessels after filling the VAPA sac to maximize the chance of bleeding cessation. Reflux and embolization of the proper hepatic artery were prevented by the covered stent. Post-embolization CBCT demonstrated complete opacification of the pseudoaneurysm with reflux of glue cast into multiple arterial feeders as well as a cessation of active extravasation [Figure 4c]. The patient’s follow-up CTA 10 days later demonstrated complete exclusion of the pseudoaneurysm and peripancreatic collateral with a glue cast. As of the most recent follow-up clinic visit at 42 months post-procedure, contrast-enhanced CT demonstrated a glue cast in the involuted VAPA and a patent stent, and there were no further episodes of gastrointestinal (GI) bleeding or adverse vascular outcomes [Figure 4d].

- A 42-year-old male status post-whipple pancreaticoduodenectomy presented with hematemesis, hematochezia, and hemorrhagic shock on postoperative day 24. (a) Initial axial arterial phase computed tomography (CT) demonstrated a pseudoaneurysm (yellow) arising from the peri-pancreatic branches of the common hepatic artery. (b) A digital subtraction angiogram from the celiac artery confirmed the location of the pseudoaneurysm (yellow), along with visualization of surrounding vasculature including the common hepatic artery (green), peripancreatic feeding vessels (blue), surgical gastroduodenal stump (red) and splenic collaterals (pink). (c) The splenic collateral providing retrograde filling of the pseudoaneurysm is embolized with coils. (d) Digital subtraction angiography confirmed the coil placement with reduced flow. However, the patient remained in hemorrhagic shock so repeat endovascular intervention was attempted. (e) Digital subtraction angiogram demonstrated the placement of covered stents to exclude small branches of the common hepatic artery (green) and gastroduodenal stump (red). Additional anatomy is noted due to the complexity of the case showing a mildly enlarged peripancreatic feeder (blue), the left gastric artery (pink) and splenic artery (purple). (f) Unfortunately, post-embolization axial arterial phase CT demonstrated persistent pseudoaneurysm (yellow) with active extravasation (orange).

- A 42-year-old male status post Whipple pancreaticoduodenectomy presented with hematemesis, hematochezia, and hemorrhagic shock on post operative day 24. The previously placed stents were used as a fluoroscopic landmark for the placement of a Chiba needle utilizing both (a) Anterior-Posterior (AP) and (b) oblique views. (c) Post-embolization cone-beam computed tomography demonstrated an appropriate glue cast of the vessels without evidence of persistent pseudoaneurysm. (d) Axial oral contrast computed tomography at 10 days follow-up revealed no further evidence of the pseudoaneurysm.
Case #3
A 27-year-old female with a medical history of chronic pancreatitis, morbid obesity, heavy alcohol dependence, and cirrhosis presented to the emergency department with abdominal pain, hematemesis, and melena. CTA on admission revealed a 2.6 cm pseudoaneurysm arising from a branch of the GDA [Figure 5a]. An angiogram was performed demonstrating the filling of the VAPA from a superior branch of the GDA [Figure 5b and c]. Endovascular coil embolization of the superior GDA branch was performed; post-embolization angiograms demonstrated no further flow to the pseudoaneurysm [Figure 5d and e]. However, the patient continued to experience persistent, severe epigastric pain. CTA performed at 2 weeks post-procedure demonstrated a persistent pseudoaneurysm with interval decrease in size [Figure 5f]. A distinct arterial feeder was not well-delineated on the CTA. Given that the antegrade approach to the VAPA was blocked by prior embolization coils and the unclear residual supply to the VAPA, the decision was made to pursue percutaneous glue embolization. CBCT with I-Guide software was used for targeting the posterior to the previous coil pack. A 21-gauge AccuStick™ II Introducer System needle (Boston Sciences Corporation, Natick, Massachusetts, USA) was advanced into the pseudoaneurysm sac under fluoroscopy confirming the position with multiple obliques and I-guide [Figure 6a-c]. Embolization was performed using 3 cc of a 3:1 ratio of lipiodol: n-BCA glue mixture. Due to the lack of a clear feeding vessel, embolization included filling the VAPA sac with an attempt to reflux into the wispy proximal feeding vasculature. The embolization endpoint was when the smaller network of wispy feeders was opacified with glue. The patient’s epigastric pain significantly improved postoperatively; follow-up CT performed at 2 months post-repeat embolization demonstrated complete embolization of the pseudoaneurysm with a glue cast in the feeding vessels [Figure 6d]. As of her most recent clinical follow-up, 6 months post-procedure, the patient denied any further episodes of gastrointestinal bleeding.

- A 27-year-old female presented with abdominal pain, hematemesis, and melena. (a) Axial IV contrast computed tomography (CT) demonstrated a large pseudoaneurysm (yellow) located proximally off a branch of the gastroduodenal artery (GDA). (b) Initial digital subtraction angiogram from the GDA demonstrated a faint blush at a superior branch of the GDA (red), which when correlated with the CT was suggestive of the pseudoaneurysm (yellow). The superior PDA (green) is noted as an alternative possible origin of the pseudoaneurysm (yellow), since it was unclear on the initial angiogram. (c) Microcatheter selection of a small superior branch of the GDA confirmed the vessel was supplying the pseudoaneurysm. Post-coil embolization digital subtraction angiograms from the (d) superior mesenteric artery and (e) celiac arteries revealed no further pseudoaneurysm filling. However, the patient continued experiencing abdominal pain, and the 2-week follow-up axial IV contrast CT (f) revealed a persistent pseudoaneurysm (yellow) with decreased size.

- A 27-year-old female presented with abdominal pain, hematemesis, and melena. (a) An AccuStick needle was advanced under fluoroscopy utilizing I-Guide software and the previously placed coils as a landmark. (b) Fluoroscopy demonstrated appropriate injection of n-butyl cyanoacrylate glue with filling of proximal small vasculature. (c) Cone-beam computed tomography (CBCT) confirmed the glue cast with the exclusion of the small proximal feeders. (d) Coronal venous phase computed tomography at the 2-month follow-up demonstrated an appropriate glue cast without further evidence of pseudoaneurysm.
Case #4
A 58-year-old male with a medical history of chronic pancreatitis and pseudocyst formation status – post-distal pancreatectomy complicated by a known pancreatic leak presented 14 days postoperatively with hypotension and a significant drop in hemoglobin. CTA revealed peripancreatic fluid and a small pseudoaneurysm in the surgical bed [Figure 7a]. The patient underwent two diagnostic angiograms which were negative due to the intermittent nature of the bleed. However, due to persistent bleeding and the known presence of a pseudoaneurysm, a third angiogram was performed demonstrating faint opacification of a pseudoaneurysm arising from a branch of the inferior pancreaticoduodenal artery (IPDA). Catheterization of the small IPDA branch was ultimately unsuccessful due to the angle and diffuse spasm of vessels in the surgical bed [Figure 7b and c]. Using the surgical clips as fluoroscopic landmarks, a 22-gauge Chiba needle was advanced into the pseudoaneurysm under fluoroscopy. Positioning of the needle was confirmed with multiple fluoroscopic obliques [Figure 8a and b]. Embolization was performed using 2 cc of a 2:1 ratio of lipiodol: n-BCA glue mixture, which was injected to fill the VAPA sac with the glue cast. The embolization endpoint was determined to be after the flow of the glue was seen in the feeding vasculature. Post-embolization angiograms demonstrated opacification of the VAPA with a glue cast. Additional superior mesenteric artery and celiac angiograms were negative for filling of the VAPA and active extravasation [Figure 8c and d]. The patient’s GI bleeding resolved after the procedure; however, the continued pancreatic leak and multifactorial medical problems continued resulting in multi-organ failure 2 weeks post-procedure.

- A 58-year-old male status – post-distal pancreatectomy 14 days postoperatively with a drop in hemoglobin and hypotension. (a) Initial axial venous phase computed tomography demonstrated a small pseudoaneurysm (yellow) in the surgical bed. (b) Digital subtraction angiogram from the superior mesenteric artery (red) revealed that the pseudoaneurysm (yellow) was arising from the inferior pancreaticoduodenal artery (IPDA) (green). (c) Digital subtraction angiogram following the selection of the IPDA (green) confirmed the location of the pseudoaneurysm (yellow). Due to the significant tortuosity of the vasculature, attempts at further navigation to the pseudoaneurysm were unsuccessful.

- A 58-year-old male status-post distal pancreatectomy 14 days postoperatively with drop in hemoglobin and hypotension. (a) Using the location of the surgical clips as landmarks, a Chiba needle was inserted into the pseudoaneurysm. (b) The pseudoaneurysm was embolized under fluoroscopy with n-butyl cyanoacrylate glue seen filling the pseudoaneurysm and proximal vasculature. Post-embolization digital subtraction angiogram from the (c) superior mesenteric artery and (d) celiac artery demonstrated no further filling of the pseudoaneurysm.
DISCUSSION
Current literature suggests that endovascular intervention should be considered as the first-line treatment of visceral aneurysms and pseudoaneurysms with surgical operation reserved for cases where an endovascular approach is not favorable.[7,14] Surgical technique may be preferred in cases of significant vessel tortuosity, failed endovascular embolization, or a high risk of end-organ ischemia with sacrifice of the vessel due to the location of the pseudoaneurysm.[8] Conversely, a surgical approach may be less favorable if the anatomical location is not suitable for surgical access or if the patient is not a safe surgical candidate.[7] In cases with a combination of poor candidacy for endovascular and surgical treatment such as the clinical vignettes presented in this study, a percutaneous approach for glue embolization is a viable treatment option.
The cases presented in this series highlight that pseudoaneurysms are at a high risk of recurrent bleeding and demonstrate that many patients undergoing treatment for a VAPA have comorbidities or tenuous hemodynamic status, lending to poor surgical candidacy.[3] In addition, recurrent bleeds often necessitate repeat intervention during which prior endovascular embolization can limit future transcatheter approaches. Although endovascular intervention is generally effective,[14] operators must have viable alternative treatment options in these emergent cases. Our cases demonstrate the successful use of percutaneous glue embolization in the treatment of VAPAs and add to a growing body of evidence supporting the use of this technique. Two relatively larger retrospective international studies have suggested that percutaneous glue embolization is a safe and effective alternative for patients who fail endovascular treatment.[12,13] One such study demonstrated the use of percutaneous glue embolization as a primary treatment option in a patient population of mixed visceral and superficial pseudoaneurysms.[12]
While the current evidence points to the significant utility of the procedure, more investigation is needed to truly evaluate the efficacy and safety of the percutaneous glue embolization technique in comparison to traditional methods such as transcatheter coil embolization. One significant factor, apart from VAPA incidence, limiting further research on this technique is the unfamiliarity of the procedure, which prevents the use of n-BCA glue outside of larger academic hospitals with physicians who have experience with the technique. Widespread physician training and continued advances in targeting guidance technology may increase utilization and improve the efficacy of this procedure.
In each of our cases, endovascular treatment was considered as the primary choice for intervention. However, persistent symptoms, challenging anatomy, and/or residual inflow to the pseudoaneurysm necessitated further treatment. The presence of prior embolic agents preventing access to the pseudoaneurysms necessitated navigating a new endovascular route or utilizing non-endovascular techniques. In our cases, a new route to the pseudoaneurysm was not successfully achieved due to tortuous anatomy and/or jailed out VAPA (Case #2, #3, and #4) and vessels that were unable to be sacrificed due to vital organ supply (Cases #1). Without the option for endovascular treatment, a multi-disciplinary decision was made to pursue percutaneous glue embolization considering the poor surgical candidacy of each patient. These cases demonstrate that percutaneous glue embolization may be a safe and effective technique as each patient had persistent embolic opacification of the pseudoaneurysms on the first follow-up CTA (mean = 26.5 days) and long-term resolution of symptoms as of the most recent follow-up (mean = 15.125 months).
While the specific scenario in which an attempt at endovascular embolization has failed and the patient is a poor surgical candidate is well-demonstrated in our clinical vignettes, other factors should be considered when deciding on the use of percutaneous glue embolization. The factors that may be beneficial but not necessary for the use of percutaneous glue embolization include a narrow pseudoaneurysm neck, visible on ultrasound, and favorable anatomical location. Conversely, a wide pseudoaneurysm neck may increase the risk of distal distribution of the embolic agent leading to end-organ ischemia in arterial anatomy at risk for ischemia, which may be the most concerning complication of this procedure. However, given that upper GI anatomy is rarely at risk for ischemia due to rich collateral networks, this technique should be more heavily considered in this territory with a high likelihood of re-collateralization.
It should additionally be noted that while this manuscript only described the use of a liquid glue embolic agent, it is also feasible to achieve embolization with other agents, such as thrombin, through the percutaneous access needle.[15] At our institution, liquid glue is preferred for pseudoaneurysm filling, due to the higher risk of recanalization with thrombin use. The use of non-liquid embolic, such as coils, to directly coil the pseudoaneurysm sac through a percutaneous approach is less favorable due to the lack of a true vessel wall. Furthermore, true extinguishing of the VAPA requires reflux of the embolic into the feeding vessels, which is why this institution considers liquid embolic agents, such as n-BCA or thrombin, more useful for a percutaneous approach.
CONCLUSION
There is currently limited evidence for the efficacy of percutaneous glue embolization of visceral pseudoaneurysms. However, the results of this case series demonstrate that there may be a significant benefit to patient health in promoting the use of this technique for life-saving measures in cases of failed endovascular embolization considering the high morbidity of surgical treatment.
Acknowledgments
Dr. Jeffrey Leichter MD, Dr. Mohamed Kably MD, Dr. Hamed Jalaeian MD, and Dr. Beau Toskich, MD.
Ethical approval
The authors declare that the research study is approved by the Institutional Ethics Committee at University of Miami Human Subject Research Office, Eprost Number: 20070111, dated 4/12/2007.
Declaration of patient consent
Patient’s consent was not required as the patient’s identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Treatment of iatrogenic false aneurysms. J Am Coll Surg. 2003;197:293-301.
- [CrossRef] [PubMed] [Google Scholar]
- Pseudoaneurysms and the role of minimally invasive techniques in their management. Radiographics. 2005;25(Suppl 1):S173-89.
- [CrossRef] [PubMed] [Google Scholar]
- Rupture risk and etiology of visceral artery aneurysms and pseudoaneurysms: A single-center experience. Vasc Endovascular Surg. 2016;50:10-5.
- [CrossRef] [PubMed] [Google Scholar]
- Visceral and peripheral arterial pseudoaneurysms. AJR Am J Roentgenol. 2005;185:741-9.
- [CrossRef] [PubMed] [Google Scholar]
- Endovascular treatment of visceral and renal artery aneurysms. J Vasc Interv Radiol. 2011;22:1246-53.
- [CrossRef] [PubMed] [Google Scholar]
- Interventional radiology in the management of visceral artery pseudoaneurysms: A review of techniques and embolic materials. Korean J Radiol. 2016;17:351-63.
- [CrossRef] [PubMed] [Google Scholar]
- Open versus endovascular treatment of visceral artery aneurysms and pseudoaneurysms. Ann Vasc Surg. 2016;35:1-8.
- [CrossRef] [PubMed] [Google Scholar]
- Is open repair still the gold standard in visceral artery aneurysm management? Ann Vasc Surg. 2011;25:936-46.
- [CrossRef] [PubMed] [Google Scholar]
- CBCT-based image guidance for percutaneous access: Electromagnetic navigation versus 3D image fusion with fluoroscopy versus combination of both technologies-a phantom study. Cardiovasc Intervent Radiol. 2020;43:495-504.
- [CrossRef] [PubMed] [Google Scholar]
- Glue embolization of a ruptured celiac trunk pseudoaneurysm via the gastroduodenal artery. Eur Radiol. 2000;10:1335-7.
- [CrossRef] [PubMed] [Google Scholar]
- Visceral artery aneurysms: Diagnosis and percutaneous management. Semin Intervent Radiol. 2009;26:196-206.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous glue embolization as a primary treatment for visceral pseudoaneurysms. Minim Invasive Ther Allied Technol. 2020;29:170-6.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous direct puncture and embolization of vascularly inaccessible abdominal visceral pseudoaneurysms: A single-center experience and literature review. J Chin Med Assoc. 2022;85:240-5.
- [CrossRef] [PubMed] [Google Scholar]
- The endovascular management of visceral artery aneurysms and pseudoaneurysms. J Vasc Surg. 2007;45:276-83. discussion 83
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous thrombin embolization of a pancreatico-duodenal artery pseudoaneurysm after failing of the endovascular treatment. World J Radiol. 2014;6:629-35.
- [CrossRef] [PubMed] [Google Scholar]






