Translate this page into:
Thermal ablation for local control of lung metastases and its effect on pulmonary function

*Corresponding author: Daniel Hyeong Seok Kim, Department of Radiological Sciences, David Geffen School of Medicine at University of California, Los Angeles, United States. dhkim@mednet.ucla.edu
-
Received: ,
Accepted: ,
How to cite this article: Kim DH, LeMaster WB, Suh R. Thermal ablation for local control of lung metastases and its effect on pulmonary function. Am J Interv Radiol. 2024;8:1. doi: 10.25259/AJIR_34_2023
Abstract
Image-guided thermal ablation in the lung has consistently demonstrated preservation of lung function without permanent decline following treatment compared to other local treatment options, specifically surgical intervention or stereotactic radiation therapy. Here, we report a case of a 68-year-old female with mesonephric adenocarcinoma of the uterus metastatic to the lung, treated with primarily thermal ablation to manage her lung tumor burden. The patient underwent a hysterectomy and wedge resection of the left lower lobe in addition to first-line chemotherapy. To reduce the total lung tumor burden, in the absence of other more effective therapies and to strategically eradicate ultra-central lung tumors, the patient underwent multiple ablative therapies. In total, she underwent 45 ablation sessions, of which 42 were cryoablation with the rest conducted with microwave ablation, two stereotactic body radiation therapies, and one brachytherapy for 75 lung metastases. Pulmonary function tests were conducted before the start of serial ablation treatments and measured again after 32 ablation sessions which revealed minimal change in pulmonary function parameters while maintaining adequate functional status. We highlight the potential benefit of ablative therapies regarding pulmonary function compared to other local treatment options for metastatic lung cancer.
Keywords
Cryoablation
Lung metastases
Microwave ablation
Pulmonary function
Thermal ablations
INTRODUCTION
While the lungs are one of the most prominent sites for metastatic disease, local treatment of select cases of metastatic disease may prolong survival and palliate symptoms, especially in patients with oligometastatic disease with five and fewer metastases, control of the primary site, long disease-free interval, and biologic favorability.[1] Options for local therapies include surgery, stereotactic body radiation therapy, or percutaneous ablations. Although surgical resection remains the most desirable option for patients with limited pulmonary metastases, it is known to significantly decrease lung function parameters. On the other hand, image-guided percutaneous thermal ablation of lung tumors is a safe and effective treatment for select patients with primary and secondary lung tumors.[1,2] Lungs in particular provide a favorable environment for computed tomography (CT)-guided ablations as there are marked contrast ratios between the tissue of the targeted tumor, aerated lung, and the ablation probe.[3] Some intrinsic advantages of percutaneous ablative therapies include their minimally invasive nature, reduced cost, and the ability to be performed in an outpatient setting. Particularly, the limited effect on pulmonary function has been one of the highly regarded benefits of image-guided ablations.[4] Thermal ablation is efficacious in various tumor types, but the majority of recent trials have shown favorable outcomes for colorectal cancer.[5,6] There exist three types of thermal ablation energies, including radiofrequency ablation (RFA), microwave ablation (MWA), and cryoablation. Specifically, cryoablation is known to better preserve the collagenous architecture of the tracheobronchial tree, provide an analgesic effect due to the cooling system, and real-time visualization of the zone of necrosis as the ice ball is readily demarcated on CT. Risks of percutaneous lung ablation include potential adverse events including pneumothorax, pleural effusion, and more commonly cryoablation, hemorrhage, or possibly hemothorax, which mostly are all self-limited but could require interventions for management. In a trial of 169 lung cryoablations, pneumothorax occurred in six patients (3.6%) of which five required chest tubes, one pleural hemorrhage (0.6%), one hypoxic event (0.6%), and one gas embolism which resolved within 24 h without any sequelae (0.6%).[5]
In this case report, we describe a patient who was diagnosed with mesonephric adenocarcinoma and was treated with systemic chemotherapy and subsequently found to have pulmonary metastases in numbers beyond the accepted definition of oligometastatic disease. The patient was treated with multiple ablations for the metastatic nodules along with brachytherapy and stereotactic body radiation therapy (SBRT) for two sites at a central location within the right lower lobe and near the center of the diaphragm. In addition, the pulmonary function tests (PFTs) before and after the series of ablations are compared. We report this case to highlight the limited effect of multiple ablation treatments on pulmonary function and support it as safe and effective for reducing lung tumor burden in the absence of more effective therapies in patients with slowly progressive indolent lung metastases.
CASE REPORT
A 68-year-old female with a history of mesonephric adenocarcinoma of the uterus treated with hysterectomy and chemotherapy presented for CT-guided ablative therapy of multiple pulmonary metastases. The patient originally presented in 2007 to an outside facility with postmenopausal vaginal bleeding and underwent hysteroscopy and was diagnosed with mesonephric adenocarcinoma of the uterus. At the time of presentation, no metastases were noted. Molecular testing revealed that the sampled tumor was epidermal growth factor receptor wild-type negative, BRAF wild-type negative, ERCC low, and human epidermal growth factor receptor 2/neu negative. Her cyclooxygenase-2 was positive at 30%, KI-67 high at 42%, PAKT positive at 70%, and PDGFRA negative. She was PDGFRB negative, P10 positive in 60% of cells, vascular endothelial growth factor positive in 40% of cells, and PI3K normal. She did well following the hysterectomy but presented again in 2010 with a cough and was found to have multiple small pulmonary nodules, measuring up to 2.2 cm, on chest imaging. Positron emission tomography (PET)/CT revealed numerous hypermetabolic pulmonary nodules, the largest of which with increased uptake (SUV 11.3) was biopsied and diagnosed as metastatic mesonephric adenocarcinoma. She subsequently underwent chemotherapy with 9 cycles of carboplatin and paclitaxel with mild improvement in the size and number of her pulmonary metastases. She underwent wedge resection of the left lower lobe as it was the location of the most significant disease burden. She underwent another 9 cycles of maintenance paclitaxel but continued to demonstrate new and recurrently enlarging pulmonary metastases, but remained asymptomatic. She was then referred to our Interventional Radiology department to consider image-guided ablation of pulmonary metastases for palliation given the lack of other acceptable treatment options and the slow but continuous progression of her lung tumor burden. Throughout the course of ablative therapies, the patient remained asymptomatic. Ultimately with accelerated disease progression, during which time ablative therapies were deemed not to be an option, she was started on immunotherapy in October 2018 without much benefit and subsequently developed a malignant right pleural effusion.
The patient underwent her first cryoablation of a right lower lobe nodule in January 2014. Given her significant disease burden at presentation and ongoing progression, she underwent several ablations each year since then – totaling 75 lung metastases, with a mean size of 0.9 ± 0.5 cm for targeted lesions, treated with 45 ablations, one brachytherapy, and two stereotactic body radiation therapy (SBRT) sessions [Table 1]. Of the total ablations, 42 were cryoablations and the remaining three were conducted with MWA [Figure 1]. Lesions that were 1 cm or greater were chosen for ablation. Other smaller lesions that were near the targeted site were also included during the same session. In addition, the location of the lesion where it would require anatomic resection was prioritized to preserve the lung. The patient also received brachytherapy for her left lower lobe basilar segmental nodal metastasis with posterior basal segmental airway involvement. In addition, she received SBRT for two sites at the central location within the right lower lobe and the central diaphragm. No lesions required repeat treatments. Follow-up CT scans were obtained every 6 months [Figure 2]. Post-ablation complications were mostly mild consisting of small pneumothorax or effusion which self-resolved or only required manual aspiration during the end of the procedure. There were two instances of small, self-limited hemothoraces. There were only three instances when the patient required a chest tube for unresolving or expanding pneumothorax which required overnight hospitalization.
| Date | Nodule size (cm) | Location | Treatment |
|---|---|---|---|
| 1-14-2014 | 1.2×0.9 | Lung – Right lower lobe | Cryoablation |
| 2-10-2014 | 1.5×1.2 | Lung – Right lower lobe | Microwave |
| 3-10-2014 | 1.1×1.0 1.0×0.8 |
Lung – Left lower lobe | Cryoablation |
| 4-7-2014* | 0.8 0.8 |
Lung – Left upper lobe | Microwave |
| 4-28-2014 | 0.9 0.6 |
Lung – Right lower lobe | Cryoablation |
| 5-19-2014 | 0.6×0.5 0.8×0.8 |
Lung – Right lower lobe | Cryoablation |
| 6-16-2014 | 0.3 0.4 0.3 0.4 0.7 |
Lung – Right upper lobe | Cryoablation |
| 7-21-2014 | 0.8×0.7 | Lung – Left upper lobe | Microwave |
| 10-15-2014 | 0.9×0.8 | Lung – Left upper lobe | Cryoablation |
| 11-26-2014 | 1.1×0.9 | Lung – Right lower lobe | Cryoablation |
| 1-12-2015 | 0.8×0.7 | Lung – Left upper lobe | Cryoablation |
| 2-9-2015 | 0.8×0.7 0.6×0.5 |
Lung – Right lower lobe | Cryoablation |
| 3-9-2015 | 1.2×1.0 | Lung – Left lower lobe | Cryoablation |
| 5-4-2015 | 0.8×0.7 0.6×0.6 |
Lung – Right upper lobe | Cryoablation |
| 6-1-2015 | 0.7×0.6 | Lung – Right middle lobe | Cryoablation |
| 7-13-2015 | 0.9×0.6 0.7×0.7 |
Lingula Lung – Left upper lobe |
Cryoablation |
| 8-17-2015 | 1.4×1.1 0.6 |
Lung – Left upper lobe | Cryoablation |
| 9-21-2015 | 0.9×0.6 0.9×0.6 |
Lung – Right upper lobe | Cryoablation |
| 10-19-2015 | 1.2×1.1 | Lung – Left upper lobe | Cryoablation |
| 11-23-2015 | 0.7×0.5 0.7×0.6 0.4×0.3 0.5×0.5 0.4×0.4 |
Lung – Right upper lobe | Cryoablation |
| 12-21-2015 | 0.8 0.6 |
Lung – Left upper lobe | Cryoablation |
| 1-25-2016 | 0.7 | Lung – Left lower lobe | Cryoablation |
| 2-29-2016 | 2.0×1.6 0.7×0.5 |
Lung – Right lower lobe | Cryoablation |
| 4-11-2016 | 0.9 | Lung – Right upper lobe | Cryoablation |
| 5-9-2016 | 0.8 | Lung – Left upper lobe | Cryoablation |
| 6-29-2016 | 0.8×0.6 1.0×0.9 |
Lung – Right lower lobe | Cryoablation |
| 8-15-2016* | 0.8×0.6 0.7×0.6 0.4×0.4 0.4×0.4 0.2×0.2 |
Lung – Right upper lobe | Cryoablation |
| 10-12-2016 | 0.9×0.5 | Lung – Right lower lobe | Cryoablation |
| 11-28-2016* | 1.4×1.2 | Lung – Left lower lobe | Cryoablation |
| 12-19-2016 | 2.0×1.9 | Lung – Right lower lobe | Cryoablation |
| 1-7-2017 | 2.0×1.9 | Lung – Right lower lobe | SBRT |
| 2-13-2017 | 1.2×0.9 1.1×0.7 |
Lung – Right upper lobe | Cryoablation |
| 3-13-2017 | 0.8 | Lung – Left upper lobe | Cryoablation |
| 4-17-2017 | 0.9×0.7 | Lung – Left lower lobe | Cryoablation |
| 6-19-2017 | 0.8×0.7 | Lung – Right lower lobe | Cryoablation |
| 7-24-2017 | 0.8 | Lung – Right lower lobe | Cryoablation |
| 9-18-2017 | 0.7×0.6 | Lung – Right upper lobe | Cryoablation |
| 11-13-2017 | 0.7×0.6 | Lung – Right upper lobe | Cryoablation |
| 1-8-2018 | 1.2 | Lung – Right lower lobe | Cryoablation |
| 2-12-2018 | 0.9×0.6 0.6×0.5 |
Lung – Left lower lobe | Cryoablation |
| 4-2-2018 | 1.1×1.1 | Lung – Right lower lobe | Cryoablation |
| 5-21-2018 | 0.8×0.7 | Lingula | Cryoablation |
| 7-9-2018 | 3.3×1.1 | Lung – Right lower lobe | Cryoablation |
| 8-13-2018 | 5.1×2.0 | Lung – Right lower lobe | SBRT |
| 9-17-2018 | 1.5×0.9 | Lung – Left upper lobe | Cryoablation |
| 9-25-2018** | 3.3×3.2 | Lung – Left lower lobe | Brachytherapy |
| 10-22-2018 | 2.8×2.8 | Lung – Left upper lobe | Cryoablation |
| 11-6-2018 | 1.1×1.1 2.8×1.2 |
Lung – Right lower lobe Lung – Right posterior costal (pleural) |
Cryoablation |
SBRT: Stereotactic body radiation therapy. *Interventions required after procedure, **Brachytherapy was conducted by placing a coaxial needle for brachytherapy catheters into the left lower lobe and left lower lobe basilar segmental nodal metastasis without complications
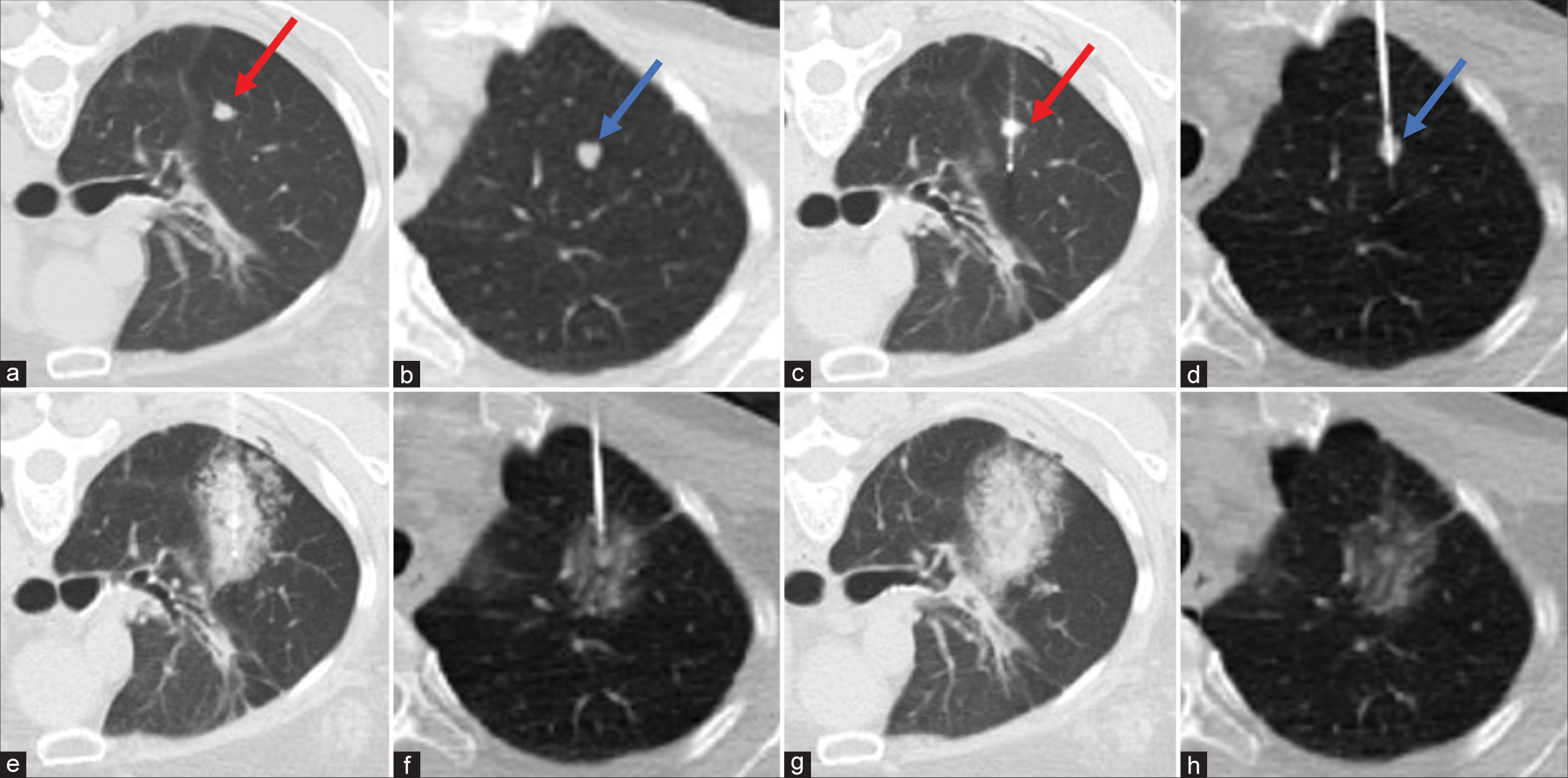
- A 68-year-old female with a history of mesonephric adenocarcinoma of the uterus presenting for computed tomography (CT)-guided ablative therapy of multiple pulmonary metastases. (a and b) Show 0.7 cm right upper lobe nodule (red arrow) and 0.8 cm left upper lobe nodule (blue arrow), respectively. (c and d) Show placement of the cryoprobe (red arrow) and microwave ablation probe (blue arrow) through the nodules, respectively. (e) Demonstrates cryoablation thaw phase which delineates the ice ball extent. (f) Intra-ablation CT shows the microwave ablation zone encompassing the target tumor. (g and h) Demonstrates post-cryoablation and microwave ablation CT, respectively.

- (a) Computed tomography (CT) of 1.2 cm right lower lobe tumor before cryoablation. (b) Immediately after cryoablation with 3, 7, and 10 min freeze with 3 min passive thaw in between with final ice ball size of 4.7 × 3.9 cm. (c) Ablation zone after 1-month post-cryoablation. (d) Ablation zone after 6-month post-cryoablation. (e) Ablation zone after 1 year post-cryoablation.
In addition, her pulmonary function test (PFT) in May 2011, before the ablative therapies but after her lung resection, reported forced vital capacity (FVC) of 3.15 L (118%), forced expiratory volume in 1 s (FEV1) of 2.25 L (110%), and diffusing capacity of the lungs for carbon monoxide (DLCO) of 13.90 mL/min/mmHg (71%). Following a sequence of 32 ablations, in April of 2017, the patient’s PFT reported FVC of 2.59 L (105%), FEV1 of 1.82 L (98%), and DLCO of 12.12 mL/ min/mmHg (93%). Overall, FEV1/FVC showed mild changes and improved diffusion capacity following serial ablations [Table 2].
| 5/2011 | 4/2017 | |
|---|---|---|
| FVC, L (% predicted) | 3.15 (118) | 2.59 (105) |
| FEV1, L (% predicted) | 2.25 (110) | 1.82 (98) |
| FEV1/FVC (% predicted) | 71 (93) | 70 (92) |
| TLC, L (% predicted) | 4.92 (111) | 4.18 (94) |
| DLCO, mL/min/mmHg (% predicted) | 13.90 (71) | 12.12 (93) |
FVC: Forced vital capacity, FEV1: Forced expiratory volume in 1 s, TLC: Total lung capacity, DLCO: Diffusing capacity of the lungs for carbon monoxide
DISCUSSION
Lungs are among the most common organs affected by metastatic disease of any origin along with the liver. While systemic therapy for the treatment of pulmonary metastases is increasingly efficacious, many patients experience progression of disease during or after treatment. In addition, some patients are poor surgical candidates for their metastases due to disease burden, poor lung function, or other comorbidities. Given that most patients with metastatic disease will have a progression of metastases, with only 27% reaching long-term disease control, lung-sparing techniques for local control, or metastasectomy, would be beneficial, since patients may require repeat procedures over time.[7] Conventionally, patients with oligometastatic disease would be committed to surgical resection and/or radiation therapy for local control when required. However, with continuously emerging safety and efficacy data of ablative techniques, image-guided thermal ablation is becoming increasingly utilized in this patient population as a form of local control either as stand-alone or in combination with systemic therapy.[1,2] This patient presented with mesonephric adenocarcinoma, a rare cancer that has no consensus on treatment options as some present with indolent course and high recurrence and others with aggressive clinical course. She underwent left lower lobe wedge resection to decrease tumor burden and had 18 cycles of chemotherapy in total but still had persistent and progressive disease. In addition, she was found to have no actionable mutation as her lung nodule was biopsied on her 36th ablation session. Although her functional status was adequate and remained asymptomatic, surgical interventions were unlikely to resolve her significant disease burden without exposing her to an unacceptable risk of complications and reduction in lung function. For this reason, to address her chronic progression of disease with eventual insurmountable tumor burden in the absence of more effective therapies, image-guided thermal ablation was chosen as a more prudent means to manage her disease burden.
Ablative therapy for lung tumors, both primary and metastatic, is safe and effective. The SOLSTICE trial showed cryoablation of lung primary and secondary efficacies to be 77% and 84%, respectively, at minimum 24-month follow-up with local recurrence-free rates of 85.1% and 77.2% and overall survival rates of 97.6% and 86.6% for 1- and 2-year, respectively.[5] This is comparable to the local efficacies of SBRT showing 83–85% with 18.7–24 month follow-up.[5] The ECLIPSE trial showed a cryoablation 5-year local control rate of 79.2%, overall survival rate of 46.7%, chemotherapy-free survival rate of 35.5%, and ablation-free survival rate of 25.4%.[2] This is comparable with those associated with metastasectomy which is reported to be 33% from a meta-analysis on metastatic liver cancer to the lung.[2] In many studies, mortality is most often cancer-specific, as the patients selected for ablation often have significant comorbidities.[3]
The effect of ablation on PFT is known for both radiofrequency ablation (RFA) and MWA showing transient decline at 3 months, but showing no worsening on longer follow-up at 6 months and up to 2 years.[6,8-10] The present case suggests that the deleterious effects of multiple lung ablations on pulmonary function may be minimal over the long term as the two compared PFT tests were approximately 6 years apart. Most ablations for this case were conducted by cryoablation which is known to better preserve the lung architecture which could have contributed to the minimal impact on pulmonary function.
In addition to the preservation of lung parenchymal function, image-guided thermal ablation has been associated with the maintenance of patient quality of life. Throughout the course of the ablative therapies, the patient’s quality of life did not suffer as shown throughout her medical records and through direct verbal communication. Quality of life as measured by the Karnofsky Performance Score demonstrated clinically significant improvement of repeated cryoablation of the lung in the SOLSTICE and ECLIPSE studies.[2,5] In contrast, traditional surgical resection, and to some degree, SBRT, involves incremental loss of pulmonary parenchyma with measurable decline in pulmonary function, and in general, poorer quality of life compared to thermal ablation.[3]
CONCLUSION
Image-guided ablation of pulmonary metastases has been widely regarded as a safe and acceptable approach to patients in the setting of oligometastatic disease. Although this patient presented with a total lung tumor burden greater than oligometastases, image-guided thermal ablation, the majority of which cryoablation, was able to address her chronic disease progression, which over time would have resulted in insurmountable tumor bulk in the absence of more effective therapies, while preserving lung function and overall quality of life. This highlights the potential advantages of image-guided lung thermal ablation, compared to other local therapeutic options.
Ethical approval
The research study is approved by the Institutional Review Board at University of California, Los Angeles, number 12-001270.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Robert Suh NeuWave Medical, Speaker Boston Scientific, Ad board and speaker Ethicon, Consultant.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Alternatives to surgery for early-stage non-small cell lung cancer: Thermal ablation. Clin Chest Med. 2020;41:197-210.
- [CrossRef] [PubMed] [Google Scholar]
- The ECLIPSE study: Efficacy of cryoablation on metastatic lung tumors with a 5-year follow-up. J Thorac Oncol. 2021;16:1840-9.
- [CrossRef] [PubMed] [Google Scholar]
- Lung cancer ablation: What is the evidence? Semin Interv Radiol. 2013;30:151-6.
- [CrossRef] [PubMed] [Google Scholar]
- Society of Interventional Radiology multidisciplinary position statement on percutaneous ablation of non-small cell lung cancer and metastatic disease to the lungs: Endorsed by the Canadian Association for interventional radiology, the cardiovascular and interventional radiological society of Europe, and the Society of Interventional oncology. J Vasc Intervent Radiol. 2021;32:1241.e1-12.
- [CrossRef] [PubMed] [Google Scholar]
- Multicenter study of metastatic lung tumors targeted by interventional cryoablation evaluation (SOLSTICE) J Thorac Oncol. 2020;15:1200-9.
- [CrossRef] [PubMed] [Google Scholar]
- Response to radiofrequency ablation of pulmonary tumours: A prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study) Lancet Oncol. 2008;9:621-8.
- [CrossRef] [PubMed] [Google Scholar]
- Stereotactic body radiotherapy for multisite extracranial oligometastases. Cancer. 2012;118:2962-70.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of radiofrequency ablation of lung cancer on pulmonary function. Cardiovasc Intervent Radiol. 2012;35:860-7.
- [CrossRef] [PubMed] [Google Scholar]
- Factors associated with pulmonary function changes in patients undergoing microwave ablation for pulmonary ground-glass nodules. Technol Cancer Res Treat. 2022;21:15330338221094429.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term results of radiofrequency ablation treatment of stage I non-small cell lung cancer: A prospective intention-to-treat study. J Thorac Oncol. 2011;6:2044-51.
- [CrossRef] [PubMed] [Google Scholar]







