Translate this page into:
Measurement of Inferior Vena Cava to Shunt Distance in Deciding Access Route for Balloon-Occluded Retrograde Transvenous Obliteration Procedure: A Pilot Study

Corresponding Author: Nischal G Kundaragi, Department of Interventional radiology and Interventional Oncology, Aster CMI Hospital, New Airport Road, Bengaluru, Karnataka, 560092, India. E-mail: drngk@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Kundaragi NG, Rachapalli V, Uthappa MC. Measurement of Inferior Vena Cava to Shunt Distance in Deciding Access Route for Balloon-Occluded Retrograde Transvenous Obliteration Procedure: A Pilot Study. Am J Interv Radiol 2018, 2(16) 1-9.
Abstract
Purpose:
Why and how to decide whether femoral or jugular approach should be used for shunt catheterization for a successful balloon-occluded retrograde transvenous obliteration (BRTO) procedure.
Materials and Methods:
Sixteen patients had undergone BRTO for variceal bleeding (11 cases) and encephalopathy (5 cases) with the femoral (13) and jugular approach (5). In two patients, both femoral and jugular approaches were used. There were four failed shunt catheterizations with the femoral or jugular approach two each. In all patients, the inferior vena cava (IVC) to shunt distance (ISD) was measured on the reformatted coronal computed tomography image.
Results:
The IVC to shunt distance (ISD) was between 2.0 and 3.5 cm in 13 patients and >3.5 cm in five. Two patients were having both proximal gastrorenal and distal splenorenal shunts. The ISD was >3.5 cm in two patients with failed initial femoral approach and < 3.5 cm in two other patients with failed initial jugular approach. In each of the four failures, the alternative approach resulted in obtaining a successful BRTO.
Conclusion:
The femoral approach is recommended for catheterization of the gastrorenal shunt for BRTO when the shunt joins the renal vein within 3.5 cm from the IVC. However, when the shunt is farther than 3.5 cm from the IVC, the jugular approach is suitable for a BRTO procedure.
Keywords
Balloon-occluded retrograde transvenous obliteration procedure
Gastrorenal shunt
Inferior vena cava to shunt distance
Transfemoral
Transjugular
INTRODUCTION
Balloon-occluded retrograde transvenous obliteration (BRTO) is performed to treat failed medical and endoscopic management of bleeding gastric varices and blocks shunts causing hepatic encephalopathy.[1,2] Various transcatheter agents have been described to treat these shunts. These range from coils, plugs, sclerosants, and gelfoam. Occlusion balloons are placed in the shunt either through a femoral or jugular vein access, through which these agents are deployed. In case of sclerosants, once the foam is injected, it causes endothelial damage and thrombosis of the shunt. Femoral and jugular routes are used, with the former being preferred. Gastrorenal shunt is the most common shunt blocked during a conventional BRTO procedure, and in 90% of patients with gastric varices, it provides venous outflow. Gastrocaval shunt and transdiaphragmatic veins provide venous outflow in the remaining 10% of patients with gastric varices. Most operators use transfemoral approach and few use transjugular approach exclusively. Pre-procedural cross-sectional imaging helps to decide the approach,[3] but it can be difficult to decide which route is most suitable for the BRTO procedure. Technically difficult (instability of guide wire in shunt and longer duration between access and shunt negotiation) shunt cannulations were thought to be due to an unfavorable angle between gastrorenal shunt and renal vein. However, we propose that inferior vena cava (IVC) to shunt distance also contributes in determining the preferred route for a BRTO.
MATERIALS AND METHODS
The study was approved by the Institutional Review Board. Informed consent was obtained for the procedure in all cases. We identified 16 patients who had undergone BRTO procedures with pre-planned contrast-enhanced computed tomography (CT) from June 2015 to March 2018 and retrospectively reviewed the clinical record, imaging, biochemical parameters, and clinical outcome. In four patients, the initial approach (two femoral and two jugular) failed due to unfavorable anatomy related to the distance between the shunt and IVC. Subsequent attempts using the alternative approach were successful in all four patients.
All measurements were done before BRTO procedure, and the procedures were performed by three interventional radiologists of 15, 10, and 7 years of experience. Coronal maximum intensity projection (MIP) images were created using portal or hepatic venous phase images; either on the IntelliSpace Portal DX Server, Philips Healthcare [CAD2], or RadiAnt DICOM Viewer, Medixant, Poland. Measurements were calculated either in true coronal or oblique coronal MIP images, but both IVC and shunt had to be visualized in same plane. IVC to shunt distance (ISD) was measured from the distal aspect of renal vein IVC junction to the proximal aspect of shunt to renal vein junction (Figure 1). We did not correlate these values with the venogram images. The authors felt that this had the potential to introduce errors due to variability in defining the distal aspect of renal vein-IVC junction because of renal vein contrast mixing with infrarenal IVC unopacified blood.
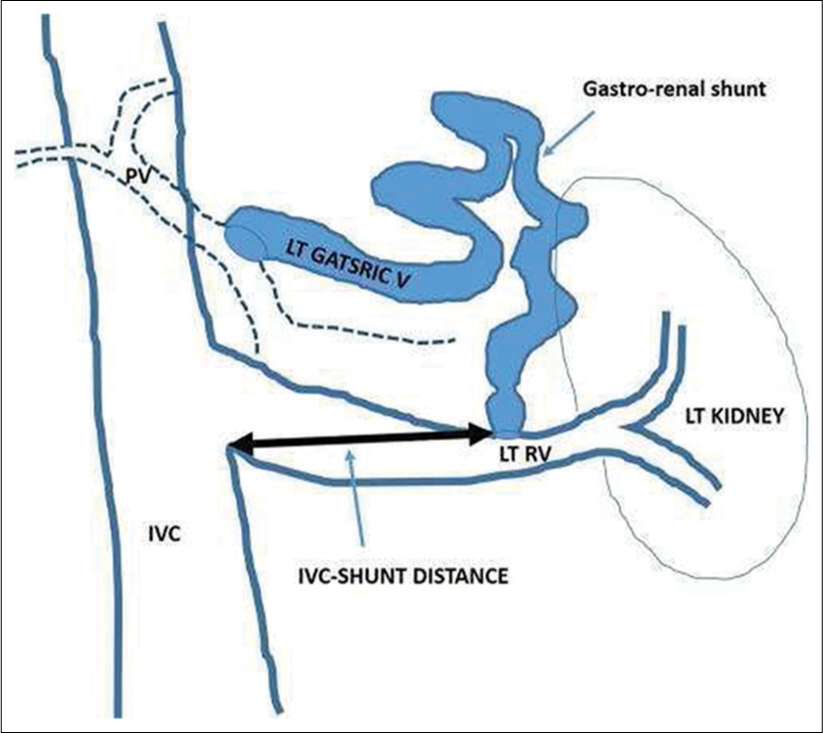
- Method to measure inferior vena cava to shunt distance.
Technique
All BRTO procedures were performed under intravenous sedation. Through femoral or jugular approach, the shunt was cannulated with a 5 Fr catheter (Cobra or MPA, Cordis, Miami Lakes, USA) and venography performed by hand injection of non-ionic contrast at a rate of 5–7 ml/s. Subsequently, the guide wire and catheter were negotiated as far as possible into the shunt. The long sheath and then a balloon catheter were tracked over a stiff wire.
In 10 cases, a 10-French Rösch-Uchida introducer sheath (Cook Inc., Bloomington, USA) and, in 2 cases, a 9-Fr 53 cm Mullins trans-septal introducer sheath (Cook Inc., Bloomington, USA) were used. In all these 12 cases, 80-cm length, 20 mm, 6 Fr occlusion balloon catheter (Serecon MP catheter II; Terumo-Clinical, Tokyo, Japan) was used. In three cases, a 12-Fr Mullins trans-septal introducer sheath (Cook, France) with 46 mm Reliant balloon (Medtronic, Minneapolis, USA) was used. In 2 of these 3 cases, 10 mm × 4 cm Rival PTA balloon (Bard Peripheral Vascular, Tempe, AZ) was also used along with Reliant balloon to block smaller shunts. In one case, 12 mm × 4 cm Rival PTA balloon (Bard Peripheral Vascular, Tempe, AZ) was used. After balloon inflation, retrograde shunt venogram was performed, and collaterals to IVC and gastric veins were identified. Microcatheter 2.7F (Progreat; Terumo, Tokyo, Japan) was used through the balloon catheter to embolize the larger collaterals with 018’ micro coils (Cook Medical Inc., Bloomington, IN) and Interlock Detachable Coils (Boston Scientific Corporation, Natick, MA, USA). Tiny collaterals without significant shunting were not embolized. One patient had two large collaterals, one from gastric vein and the other from splenic vein with multiple communications to gastric varices having common drainage into the renal vein. In this case, we embolized the splenorenal shunt with 0.035” coils through the jugular route and injected sclerosant through a Reliant balloon catheter from the femoral route, as balloon catheter negotiation through the jugular route was unsuccessful because of stiffness.
Two cases of recurrent hepatic encephalopathy had more than one shunts. Gastrorenal shunts were closer to IVC compared to splenorenal shunts. In both these patients, we used femoral and jugular approach for closer gastrorenal shunt and farther splenorenal shunts, respectively. In some patients, after accessing the shunt, part of it was embolized with coils and then BRTO performed (similar to coil assisted BRTO).
In all cases, the volume of foam sclerosant required was calculated by injecting contrast through the inflated balloon catheter, with complete opacification of shunt and gastric varices as the end point. Subsequently, foam sclerosant (3% sodium tetradecyl sulfate mixed with room air −3:2 ratio) was injected into the shunt. Volume of foam sclerosant injected ranged between 16 cc and 28 cc. After administration of sclerosant, the balloon was kept inflated for a minimum of 1½ h. Balloon was slowly deflated under fluoroscopy after confirming visible stasis of the sclerosant mixture within the shunt/varices. In some patients with non-visualization of stasis, 1–2 ml of contrast was injected to confirm thrombus formation before balloon deflation. The balloon catheter was withdrawn. Post-procedure venogram was done to rule out renal vein thrombosis.
RESULTS
Sixteen cases of BRTO were undertaken between June 2015 and March 2018. Eleven patients had gastric variceal bleeds and five patients had recurrent hepatic encephalopathy. Most of them had gastrorenal shunts from the left gastric vein to the left renal vein. Two patients with hepatic encephalopathy had more than one shunt. There was male predominance, with a male-to-female ratio of 15:1. The mean age was 70 years (range 23–80 years). Table 1 summarizes the patient details, cause for bleeding, type of shunt, IVC to shunt distance, route of access, and outcome.
| Age/sex M–Male F-Female | Reason for BRTO | Type of shunt | IVC-shunt distance (ISD) in cm | Transfemoral approach | Transjugular approach | Number of times Glide wire slipped back into renal/IVC, transfemoral approach | Result |
|---|---|---|---|---|---|---|---|
| 37 years/M | EHPVO with gastric variceal bleeding | Splenorenal shunt | 3.0 | Successful | Not attempted | One | Patient recovered completely |
| 63 years/M | Alcoholic liver Cirrhosis with gastric variceal bleeding |
Gastrorenal shunt | 2.67 | ” | Not attempted | Nil | ” |
| 65 years/M | Hepatitis B Cirrhosis with Gastric variceal bleeding |
Gastrorenal shunt | 3.50 | ” | Not attempted | Three | ” |
| 23 Years/M | EHPVO with Gastric variceal bleeding |
Gastrorenal shunt | 2.92 | ” | Not attempted | One | ” |
| 60 Years/M | Alcoholic liver cirrhosis with gastric variceal bleeding |
Gastrorenal shunt | 2.81 | ” | Not attempted | One | ” |
| 61 Years/M | NASH cirrhosis with recurrent encephalopathy | Gastrorenal shunt | 3.36 | ” | Not attempted | Two | ” |
| 57 Years/M | NASH cirrhosis with gastric variceal bleeding |
Gastrorenal shunt | 3.69 | Attempted but failed to pass balloon. | Successful | Wire was not stable to pass balloon catheter | ” |
| 66 Years/M | Hepatitis B cirrhosis with recurrent hepatic encephalopathy |
Gastrorenal shunt | 4.45 | Attempted but failed to pass balloon. | Successful | “ | Patient on follow-up>6 months. No encephalopathy. Rectal bleed due to internal hemorrhoids at 3 months |
| 46 Years/F | Idiopathic liver cirrhosis with gastric variceal bleeding | Gastrorenal shunt | 2.77 | Successful | Attempted but failed to pass balloon | Wire was stable but too much strain while passing balloon catheter | Patient died due to liver/multiorgan failure. |
| 63 Years/M | Alcoholic liver cirrhosis with gastric variceal bleeding | Two Shunts (Gastrorenal and Splenorenal) with common renal vein drainage | 3.13 | Successful BRTO done with balloon in at junction of shunts | Attempted but failed to pass balloon. Coils were put in to the splenorenal shunt | Wire was stable but too much strain while passing balloon catheter | Patient recovered completely |
| 80 Years/M | Alcoholic cirrhosis with Gastric variceal bleeding | Gastrorenal shunt | 3.37 | Successful | Not attempted | Two | ” |
| 74 Years/M | Hepatitis B cirrhosis with Gastric variceal bleeding |
Gastrorenal shunt | 2.70 | Successful | Not attempted | Nil | ” |
| 63 Years/M | Hepatitis C cirrhosis with Gastric variceal bleeding |
Gastrorenal shunt | 3.39 | Successful | Not attempted | Two | ” |
| 70 years/M | Hepatitis B cirrhosis with treated case of ruptured HCC (TACE 2 times). Recurrent encephalopathy |
Gastrorenal and splenorenal shunt | 3.13 and 5.10 | Successful for gastro--renal shunt. | Successful for splenorenal shunt. | NA | “ |
| 69 Years/M | Idiopathic liver cirrhosis with recurrent HE | Gastrorenal and two splenorenal shunts | 3.38, 5.09, and 7.55 (5.09+2.46 cm) | Successful for gastrorenal shunt. Successful for coiling of proximal splenorenal shunt, but failed for balloon placement. | Successful for distal splenorenal shunt. | Proximal splenorenal shunt. 3 times | Patient died due to sepsis and liver failure at 3 months. |
| 64 Years/M | NASH cirrhosis with Recurrent encephalopathy | Gastrorenal shunt | 3.82 | Not attempted | Successful | NA | On follow-up. Recovering well. |
BRTO: Balloon--occluded retrograde transvenous obliteration, IVC: Inferior vena cava
Twelve patients had isolated gastrorenal shunt. Two patients with refractory hepatic encephalopathy had both gastrorenal and splenorenal shunts with separate renal vein opening. One patient with refractory encephalopathy had isolated splenorenal shunt. One patient with gastric variceal bleed had both gastrorenal and splenorenal shunts with common drainage into the left renal vein. When measured on reformatted true and oblique coronal CT scan MIP images, the ISD was between 2 and 3.5 cm in 11 patients and >3.5 cm (3.7, 4.3 and 3.82 cm) in 3 patients. Two patients with more than one shunt had ISD <3.5 cm for gastrorenal shunts and >3.5 cm for splenorenal shunts.
The femoral approach was used in 11 patients, jugular approach in 3 patients, and both approaches for 2 patients. The jugular approach was successful in 3 patients with ISD >3.5 cm; however, femoral approach failed to cannulate the shunt successfully in 2 of these patients (Figures 2 and 3). In one of these patients with a longer ISD, the shunt was embolized by jugular approach first without attempting femoral approach.

- 66-year-old male, known Hepatitis B virus cirrhosis, presented with recurrent HE (a) Contrast-enhanced computed tomography coronal maximum intensity projection image in a patient with recurrent hepatic encephalopathy, who had undergone unsuccessful coil occlusion of the shunt. The gastrorenal shunt is poorly seen because of the metal artifact from the coils. Large gastric varices are seen in the gastric fundus. (b) Left renal venogram showing the patent left renal vein and previously placed coils. Balloon-occluded retrograde transvenous obliteration (BRTO) failed due to the inability to catheterize the shunt from a femoral approach. (c) Fluoroscopic image (right anterior oblique projection) taken during deflation of the balloon following coil-assisted BRTO from the jugular approach shows coils and shunt occlusion.

- 57-year-old male, known NASH cirrhosis, presented with active gastric variceal bleeding. (a) Contrast-enhanced computed tomography coronal maximum intensity projection image shows a small gastrorenal shunt with ISD of 3.69 cm and mid caval narrowing above and below the hepatic segment of inferior vena cava (IVC). Angle between shunt and renal vein is obtuse. Vertical line drawn along the IVC margin measures 4.71 cm. (b) Fluoroscopic image shows sheath in renal vein and catheter in shunt. (c) Fluoroscopic image during successful balloon-occluded retrograde transvenous obliteration demonstrating staining of shunt. Easy negotiation of balloon occlusion catheter into shunt through transjugular access.
In two patients with ISD 2.77cm (bleed) and 3.13 cm (bleed with two shunts), we attempted jugular approach initially. In the first case, we failed to pass the balloon catheter into the shunt because of significant strain on the renal vein preventing us from pushing the balloon catheter further (Figure 4). In the second case, a lateral shunt collateral from the splenic vein was embolized with coils through jugular approach; however, due to the strain on the renal vein, we were unable to pass balloon catheter into the shunt. In both these cases, femoral approach was successful.

- 46-year-old female, known idiopathic cirrhosis, presented with active gastric variceal bleeding. (a) Contrast-enhanced computed tomography Coronal maximum intensity projection, ISD ∼2.7 cm. (b) Fluoroscopic image showing 5F Cobra catheter and Amplatz wire forming large arc from jugular access. Negotiation of 6F balloon catheter was difficult and strain noted on inferior vena cava and renal vein. (c) Venogram performed after inflating balloon. No collaterals were seen. Easy negotiation of shunt vein was done through the transfemoral approach with long straight sheath support and subsequent successful balloon-occluded retrograde transvenous obliteration.
In two cases of recurrent hepatic encephalopathy with more than one shunt, femoral and jugular approaches were used for closer gastrorenal shunt and farther splenorenal shunt, respectively. One patient had two shunts, one gastrorenal and one splenorenal shunt. Gastrorenal shunt (ISD ∼3.13 cm) was accessed through femoral route and splenorenal shunt (ISD - 5.10 cm) was accessed through jugular route, and balloons were placed for embolization and successful BRTO was performed (Figure 5). Another patient had 3 shunts, one gastrorenal and two (proximal and distal) splenorenal shunts. Gastrorenal shunt (ISD - 3.38 cm) was accessed through femoral route and distal splenorenal shunt (ISD - 7.55 cm) was accessed through jugular route, and balloons were placed for embolization. However, proximal shunt (ISD - 5.09 cm) was accessed through femoral route after many unsuccessful attempts. We were unable to pass the balloon catheter due to wire instability, so we had to embolize the shunt with 4F vertebral catheter and 035’ coils (Figure 6).

- 70-year-old male, known hepatitis B virus cirrhosis, presented with recurrent hepatic encephalopathy. (a) Contrast-enhanced computed tomography coronal maximum intensity projection image showing gastrorenal and splenorenal shunts with ISD ∼3.13 and 5.10 cm, respectively. Previously, TACE was performed for ruptured segment 6 HCC with complete response to treatment (arrow). (b) Fluoroscopic image showing balloon catheters in both shunts. (c) Fluroscopic image after completion of BRTO procedure showing contrast staining of shunts.

- 69-year-old male, known idiopathic liver cirrhosis, presented with recurrent hepatic encephalopathy. (a) Contrast-enhanced computed tomography oblique Coronal maximum intensity projection (MIP) image showing gastrorenal (open arrow) and splenorenal (arrow) shunts with ISD ∼3.32 and 5.09 cm, respectively. (b) CECT oblique coronal MIP image showing proximal (arrow) and distal (arrow heads) splenorenal shunts with distance between them measuring 2.46 cm. (c) Fluoroscopic image showing two microcatheters in the gastrorenal shunt by femoral approach and distal splenorenal shunt by jugular approach. Shunt venogram performed through distal splenorenal shunt (arrow heads). Coils were placed in collateral. (d) Fluoroscopic image showing contrast staining of distal splenorenal shunt after placing coils and balloon. Proximal splenorenal shunt venogram after accessing through femoral route (arrow). Difficult cannulation by transfemoral route. (e) Inability to pass balloon catheter (due to wire instability) into the proximal splenorenal shunt; therefore, it was embolized with coils (arrow). (f) Fluoroscopic image showing two balloons in gastrorenal (open arrow) and distal splenorenal shunts and sclerosant staining of shunts.
In the remaining cases, ISD was between 2.67 cm and 3.5 cm. Balloon catheter negotiation across the renal vein into the shunt was easier with ISD <3 cm compared to a patient with ISD >3 cm (Figure 7). In cases with ISD >3 cm, the glide wire tends to slip back into IVC or renal vein (Figures 6 and 8). Once a stiff wire was negotiated into the shunt, it was easier to advance the balloon catheter into the shunt. After balloon inflation, large collaterals were identified and embolized with micro coils (4 cases of bleeds and 1 case of hepatic encephalopathy).

- 63-year-old male, known alcoholic cirrhosis, presented with active gastric variceal bleeding. (a) The ISD measures 2.67 cm on the contrast-enhanced computed tomography coronal maximum intensity projection image. (b) Fluoroscopic image taken after inflation of the occlusion balloon with contrast medium, and the injection of contrast medium fills the large shunt. A phrenic collateral vein had been embolized with coils. Note that the sheath tip is placed in the left renal vein.

- 65-year-old male, known hepatitis B virus cirrhosis, presented with active gastric variceal bleeding. (a) Contrast-enhanced computed tomography coronal maximum intensity projection image with ISD ∼3.5 cm. (b) Fluoroscopic image showing abnormal curves of 5F catheter with glide wire in distal aspect of shunt and difficulty negotiating the shunt. (c) Fluoroscopic image after balloon inflation. Sheath tip in the shunt. This case demonstrates the concept that the larger the distance of the shunt from inferior vena cava, the more difficult it is to cannulate the shunt from the femoral route.
Post-procedure, all the patients with refractory hepatic encephalopathy showed improvement. By day 3, the encephalopathy had improved, and by 1 month, reduction in serum ammonia levels was noted.
The procedure was technically successful in all the patients with no immediate procedure-related complications. With the exception of a small minor contrast leak, no major venous rupture occurred. Focal minor renal vein thrombosis occurred around the catheter in two patients, which resolved without any further intervention. All the patients were transferred to surgical ICU for post-procedure monitoring. Two patients died during follow-up: One due to liver failure 3 days after the procedure and the other due to sepsis, liver, and multiorgan failure at 3 months. All the remaining patients were subsequently discharged between post-procedure days 5 and 7.
DISCUSSION
Gastric varices are often difficult to manage and require multidisciplinary management. Gastric variceal bleeding tends to require increased blood transfusion requirements and have higher mortality rate compared to esophageal variceal bleed.[4] BRTO has become an established procedure in the management of gastric varices. It has a technical success rate of around 91%.[5]
Obtaining a stable balloon position is vital to prevent migration of the embolic agent into the renal vein. Transfemoral approach is preferred in view of “pushability” of the wire-catheter system and safety.[6] Given the variation in the availability of equipment, operators use what equipment is locally. Hence, it is important to plan the procedure, particularly the access, as jugular approach can increase the procedure time by nearly 1 h.[6]
Transfemoral catheterization of the gastrorenal shunt can be challenging. In cases when the left renal vein makes an acute angle with the IVC, the transjugular approach is more favorable.[6,7] If the shunt is relatively perpendicular to the renal vein, a femoral approach is recommended. If the angle is relatively acute with the renal vein, a transjugular approach is recommended.[6] Saad et al. stated that the further the distance of the shunt from the IVC, the greater the preference for the transjugular approach.[6] However, they did not quantify the distance between the shunt and IVC. According to Gwon et al., transjugular approach was used when the angle was acute between left renal vein and adrenal vein.[8]
When the authors used long curved sheaths, they considered the angle between IVC and renal vein and between the renal/adrenal vein and shunt to prevent failed catheterization or instability of the balloon. However, after using long, curved support sheaths, we feel ISD matters more than the angles as sheaths allow greater stability, regardless of angles. Graphical representation shows transfemoral and transjugular approach in passing wire and catheters with strain placed on the renal vein (Figure 9a-d).

- Effect of ISD on sheath/wire placement through transjugular (a and b) and transfemoral (c and d) approach. Longer the ISD (double-headed arrows), easier the cannulation of shunt through jugular approach (a) and difficult through femoral approach (c) and vice versa (b and d).
An acute angle between the renal vein and shunt can make BRTO technically challenging. To overcome this problem, Shukla et al. modified the technique using two jugular sheaths. A 5-F vascular sheath was placed from the right internal jugular vein and used for injecting sclerosant into the shunt through a coaxially introduced microcatheter. A balloon occlusion catheter was placed into the shunt coaxially through another 6-F vascular sheath placed from the external jugular vein.[9] We feel that this technique should be used when microcatheter is a must for targeted embolization and balloon catheters are of smaller diameter. If a curved supporting sheath is used, an acute angle between renal vein and shunt matters less.
There is insufficient evidence to support angles determining in deciding which access route should be selected for BRTO. If adequate straight/curved supporting sheath is used during the BRTO procedure, ISD matters more than angles. Future pooled data from multiple centers can improve our understanding. Further studies are also needed to see if the ISD of 3.5cm can be used as a cut of to decide the approach for undertaking BRTO.
CONCLUSION
Pre-planning CECT with coronal MIP reformation with the measurement of the distance between the IVC and shunt can help decide whether the femoral or jugular approach should be used for BRTO.
On the basis of the technique used in our study, the jugular approach should be used when the ISD is >3.5 cm and the femoral approach when the ISD is <3.0–3.5 cm. More prospective data will help investigate our theory.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Balloon-occluded retrograde transvenous obliteration of gastric varices. AJR Am J Roentgenol. 2012;199:721-9.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of balloon-occluded retrograde transvenous obliteration of large spontaneous lienorenal shunt in patients with severe recurrent hepatic encephalopathy with foam sclerotherapy: Initial experience. J Vasc Interv Radiol. 2012;23:1200-6.
- [CrossRef] [PubMed] [Google Scholar]
- Balloon-occluded retrograde transvenous obliteration of gastric varices: Concept, basic techniques, and outcomes. Semin Intervent Radiol. 2012;29:118-28.
- [CrossRef] [PubMed] [Google Scholar]
- Balloon-occluded retrograde transvenous obliteration for gastric variceal bleeding: Its feasibility compared with transjugular intrahepatic portosystemic shunt. Korean J Radiol. 2003;4:109-16.
- [CrossRef] [PubMed] [Google Scholar]
- Balloon-occluded retrograde transvenous obliteration (BRTO): Technical results and outcomes. Semin Intervent Radiol. 2011;28:333-8.
- [CrossRef] [PubMed] [Google Scholar]
- The conventional balloon-occluded retrograde transvenous obliteration procedure: Indications, contraindications, and technical applications. Tech Vasc Interv Radiol. 2013;16:101-51.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of transcatheter embolization for hepatic encephalopathy caused by spontaneous portosystemic shunts. Intervent Radiol. 2017;2:51-8.
- [CrossRef] [Google Scholar]
- Gastric varices and hepatic encephalopathy: Treatment with vascular plug and gelatin sponge-assisted retrograde transvenous obliteration-a primary report. Radiology. 2013;268:281-7.
- [CrossRef] [PubMed] [Google Scholar]
- Balloon-occluded retrograde transvenous obliteration using a dual venous access and sheath system. Diagn Interv Imaging. 2016;97:495-8.
- [CrossRef] [PubMed] [Google Scholar]






