Translate this page into:
Extracellular matrix enterocutaneous fistula plug placement: An interventional radiology-guided procedure for fistulae refractor y to care

-
Received: ,
Accepted: ,
How to cite this article: Girardot J, Xiao N, Molina H, Resnick S. Extracellular matrix enterocutaneous fistula plug placement: An interventional radiology-guided procedure for fistulae refractory to care. Am J Interv Radiol. 2024;8:4. doi: 10.25259/AJIR_4_2024
Abstract
Objective:
Enteric fistulas are serious bowel injuries that significantly decrease patient life quality. Operative treatments neglect patients who are not surgical candidates or who have failed surgery. One non-operative method is the percutaneous placement of an extracellular matrix enterocutaneous fistula plug (ECMFP), which sits in the fistula tract and constructs a surface into which the fistula heals.
Materials and Methods:
This study included ten patients who had an ECMFP placed between June 2017 and July 2022 with follow-up through October 2022. The median patient age was 66.5 years. Fistulae origins were gastrocutaneous (n = 1), enterocutaneous (n = 4), and colocutaneous (n = 5).
Results:
Of the ten patients, fistula closure was achieved in 5 (50%). Closure occurred in three of four enterocutaneous (75%), one of one gastrocutaneous (100%), and one of five colocutaneous fistulae (20%). The median time to closure was 1 month. Successfully closed fistulae had a mean duration of existence of 4.6 months. Failed closures had a mean duration of existence of 15.3 months. Fistulae originating from a percutaneous enteric tube had success in 2 of 3 patients (66%). Fistulae due to diverticulitis did not achieve closure (0 of 3).
Conclusions:
ECMFPs are a viable treatment to consider in a patient population that has few other options. They can be useful for the closure of fistulae involving the stomach or small bowel with an existence of <1 year. Patients with fistulae of colonic origin, patients who have had fistulae for longer than 1 year, or patients whose fistulae are due to diverticulitis are less likely to see successful closure.
Keywords
Enterocutaneous fistula
Fistula
Extracellular matrix
Fistula plug
INTRODUCTION
Enteric and colonic fistulas are serious complications of bowel injury that lead to a significant decrease in patient quality of life by delaying healing, prolonging hospitalizations, and potentially leading to other issues such as necrosis or sepsis.[1] Reported mortality rates for enteric fistulae are from 10% to 30%, with sepsis as the largest contributing factor.[2-4] Catheter drainage of abscesses is reported to result in spontaneous closure of fistulae in 20–75% of cases with few other conservative measures.[2,5-7] Operative treatment of enterocutaneous fistula (ECF) has reported success rates of 80–90%.[2,8] However, these treatments neglect patients who are not candidates for surgery or those who have failed prior surgical treatment.
Many non-operative treatment options have been previously discussed in the literature.[4,8-10] One such non-operative method is the placement of an extracellular matrix enterocutaneous fistula plug (ECMFP), which sits in the fistula tract and constructs a surface into which the fistula may heal. These plugs have been evaluated for use in anal fistulae.[11-14] Following their use in anal fistulae, a new plug, the Biodesign Fistula Plug (Cook Biotech Inc, West Lafayette, Indiana, USA) was created with the specified purpose of closing ECF. A few studies have investigated their utility with these fistulae.[15-18] These studies have considered foregut fistulae, high-flow duodenal fistulae, and fistulae throughout the enteric tract. The purpose of this study was to investigate the clinical utility of ECMFPs at a single institution and to identify patient characteristics that may be indicative of plug success in fistula closure.
MATERIAL AND METHODS
Study design
This study was a retrospective single institution case series conducted with the Local Institutional Review Board approval. From June 2017 to July 2022, patients with ECFs that developed or had failed prior closure attempts were considered for placement of a Biodesign ECMFP (Cook Biotech Inc., West Lafayette, Indiana, USA) [Figure 1]. Inclusion and exclusion criteria were fairly simple. Patient fistulae could have a max diameter of seven millimeters and a max length of fifteen centimeters. The fistula tract also could not have any associated abscess cavities along its course. The requirement for consent was waived. The follow-up period was from the time of plug placement through October 2022. Follow-up visits were conducted at 2 weeks, 1 month, and 6 months post-procedure, or as requested by patients. Six months was the endpoint of the study unless further visits were requested by patients.
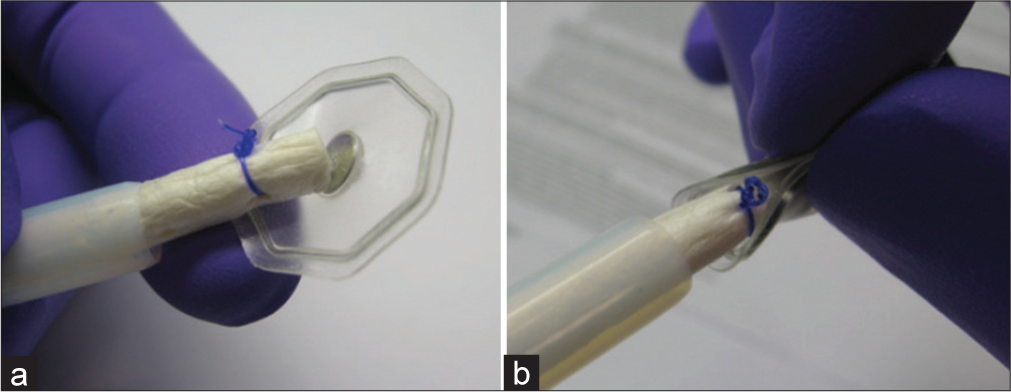
- Biodesign Enterocutaneous Fistula Plug (Cook Biotech Inc, West Lafayette, Indiana, USA). (a) Depicted is the fistula plug within its delivery sheath with the flexible footplate deployed at the tip of the plug. (b) The same fistula plug is depicted, but with the footplate flexibility demonstrated as it is meant to be able to adhere to the curvature of the bowel wall.
Patient demographics
The median patient age was 66.5 years old at the time of plug placement. There were six men and four women. Factors that may slow or decrease healing capacity were identified and included the use of corticosteroids, smoking, diabetes, and treatment with chemotherapy and radiation. Two patients were being treated with corticosteroids. Three patients were smokers, three had an extensive history of smoking, and four were non-smokers. Two patients were diabetic. No patients were being treated with chemotherapy, although two had been treated with chemotherapy in the past, and one had been treated with radiation. Full patient characteristics are listed in Table 1.
| Patient Age/Sex |
Success | Location | Fistula Origin | Duration of Fistula Existence | Time to Failure | Time to Closure | Fistula Size (mm) | Fistula Length (cm) | Complex Fistula | Steroids | Smoking | DM | Chemotherapy | Radiation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 65/F | Y | Gastrocutaneous | Percutaneous gastrostomy tube site | 1 month | N/A | 3 months, 11 days | 4 | 6.9 | N | N | Y | Y | N | N |
| 68/M | Y | Ileocutaneous | Ileostomy, drainage s/p exploratory laparotomy | 5 months | N/A | 1 months | 4 | 8.5 | Y | N | Y | N | Y | N |
| 54/M | Y | Colocutaneous | Pancreatitis (Alcohol induced) | 5 months | N/A | 9 months | 4 | 10 | N | N | N | N | N | N |
| 70/F | Y | Small bowel, non-specific | Complicated small bowel obstruction requires surgery | 6.5 months | N/A | 1 month | 4 | 13.5 | Y | N | N | Y | N | N |
| 60/M | Y | Small bowel, non-specific | Fistulizing ileal Crohn’s | 5.5 months | N/A | 1 month | 4 | 9 | N | Y | N | N | N | N |
| 70/F | N | Colocutaneous | Perforated diverticulitis s/p colectomy | 1 year 2 months | 8 months | N/A | 10 | 20 | Y | N | Y | N | Y | Y |
| 86/M | N | Colocutaneous, sigmoid | Diverticulitis | 4.5 months | 2 months for each plug | N/A | 3, 2 | 7, 10 | Y | N | Y | N | N | N |
| 69/M | N | Colocutaneous, descending/sigmoid | Diverticulitis | 1 year 2 months | 1 week | N/A | 3 | 7 | Y | Y | Y | N | N | N |
| 44/F | N | Duodenocutaneous | Liver transplant complicated by fistula | 2 years | 2 months | N/A | 3 | 4 | Y | N | N | N | N | N |
| 62/M | N | Colocutaneous, descending | Colonic interposition with Roux-en-Y colojejunostomy | 1 year 8 months | 5 days | N/A | 4 | 8.5 | Y | N | Y | N | N | N |
Complex Fistula: Fistula with side tracts, DM: Diabetes mellitus, N: No, Y: Yes, N/A: Not applicable
Fistula location and characteristics
Fistulae origins were gastrocutaneous (n = 1), enterocutaneous (n = 4), and colocutaneous (n = 5). The median fistula tract length was 8.5 cm and lengths were determined using measurements from dedicated ECF mapping procedures in advance of the plug placement procedure. For fistula mapping, the ECF tract was catheterized via the skin opening utilizing a Glidewire/Glidecatheter combination (Terumo Interventional Systems, Somerset, New Jersey, USA) and the ECF tract navigated to achieve catheter placement into the draining bowel. A Magic Torque calibrated measuring Guidewire (Boston Scientific Corporation, Marlborough, Massachusetts, USA) was placed, and a sheath was advanced over the Guidewire followed by detailed mapping of the ECF tract through pull-back contrast injection. Measurements regarding tract length and diameter were made as well as identification of any fistula tract multiplicity and/or associated abscess cavities. Inclusion and exclusion criteria for this study included a max fistula diameter of seven millimeters and a max length of fifteen centimeters. Fistulae also could not have any associated abscess cavities along their length. Based on these findings, appropriateness, or lack thereof, for ECF plug placement was determined. The number of patients excluded from this study for not having the required criteria was not tracked. If the ECF anatomy was deemed appropriate for ECF plug placement then following ECF mapping, an 8 Fr self-retaining drainage catheter was left in place within the bowel to achieve ECF tract control and guarantee ready access into the bowel at the time of subsequent ECF plug placement procedure [Figure 2]. Full fistula characteristics for each patient are listed in Table 1.
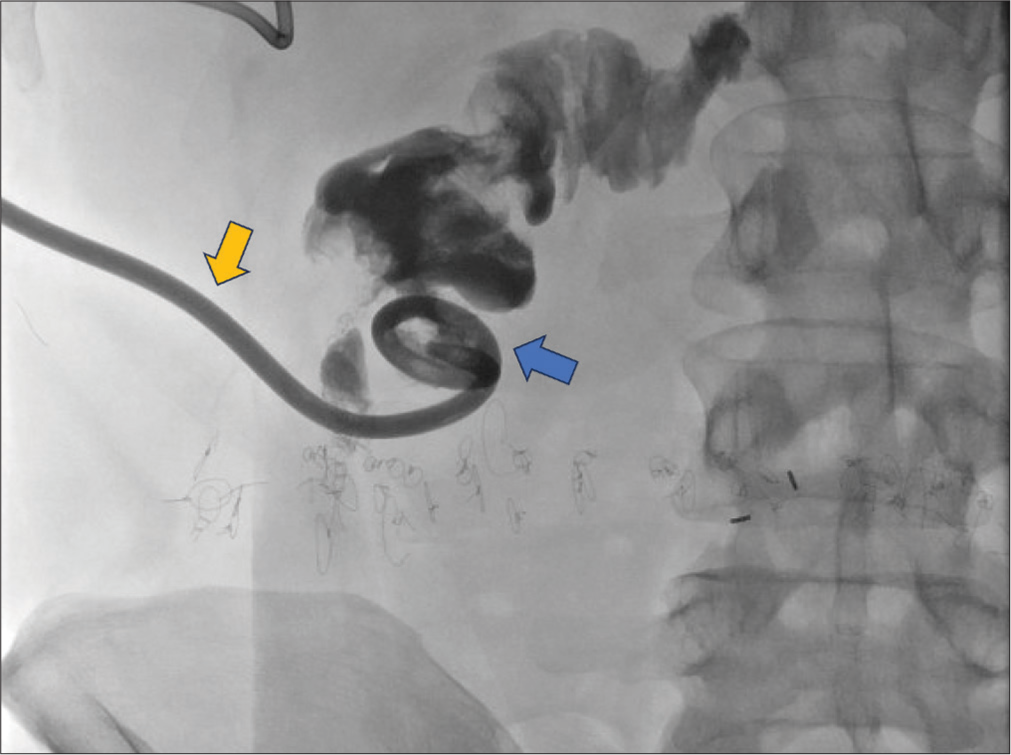
- A 60-year-old male with Crohn’s disease who presented with an enterocutaneous fistula. Procedurally obtained digital X-ray fluoroscopic image shows the placement of a self-retaining drainage catheter left in place within a small bowel (blue arrow) through the fistula tract (yellow arrow). Contrast highlights the small bowel lumen.
Procedure details
ECF plug placement procedures were performed under intravenous moderate sedation, monitored anesthesia care, or general anesthesia; the choice of anesthetic was based on patient-specific factors. Anticoagulation was not withheld from patients before the procedure. For ECFs involving the colon, a bowel preparation was variably utilized the evening preceding the plug placement procedure at the discretion of the operator. Fluoroscopy was used to assist in the insertion, advancement, and placement of all catheters, guide wires, the ECMFP, and other associated instruments. The indwelling 8 Fr catheter was removed and a stiff Amplatz (Cook Medical, Bloomington, Indiana, USA) or Lunderquist (Cook Medical, Bloomington, Indiana, USA) was placed into the bowel lumen. The sideport injection sheath was then advanced to the portion of the fistula tract communicating with the bowel. Contrast was then injected to demarcate the fistula location within the bowel. The sheath was then removed, and a cytology brush (Boston Scientific Corporation, Marlborough, Massachusetts, USA) or similar instrument was then inserted and advanced into the fistula tract. The brush was then actuated to debride the interior of the tract and remove granulation tissue. The tract was also variably flushed with hydrogen peroxide through a syringe at the discretion of the operator. The ECMFP and dilator delivery system were then prepared, and the delivery system advanced over the guide wire until the flexor sheath tip extended 1–2 cm into the bowel lumen. The guide wire was then removed, and the fistula plug was loaded into the transfer tube. The plug was then advanced out of the transfer tube into the delivery sheath until it was fluoroscopically determined to be in the tip of the delivery sheath, and the tip of the delivery sheath was determined to be 1–2 cm in the lumen of the bowel. The plug was then advanced into the bowel until the flange was deployed into the bowel lumen, and fluoroscopic imaging confirmed the full deployment of the flange. The delivery sheath was then withdrawn from the tract, and tension was applied to the plug to retract the flange against the bowel wall [Figure 3]. A Molnar disk was then secured to the skin through the exposed tether at the end of the fistula plug, and proper placement of the plug was confirmed via fluoroscopy.

- A 60-year-old male with Crohn’s disease who presented with an enterocutaneous fistula. Procedurally obtained digital X-ray fluoroscopic image shows the placement of the fully deployed extracellular matrix plug (blue arrow) through the fistula tract with a deployed flange in the small bowel lumen (yellow arrow). Contrast highlights the small bowel lumen.
Data abstraction
Descriptive statistics were used in the report of this study’s findings. Data abstraction for procedural technical success, fistula location and etiology, fistula size and length, plug size, time to fistula closure, and duration of fistula existence before plug was performed. Technical success was defined as fistula closure without leakage or later recurrence. Technical failure occurred if the fistula continued to exhibit drainage at all follow-up visits. Recurrence was defined as persistent leakage following a leakage-free period post-procedure. Follow-up visits were conducted at 2 weeks, 1 month, and 6 months post-procedure to determine recurrence.
RESULTS
Of the ten patients included in this study, fistula closure was achieved in 5 (50%). As such, the technical success rate for this procedure in this study was 50%. Closure occurred in three of four enterocutaneous (75%), one of one gastrocutaneous, and one of five colocutaneous fistulas (20%). The median time to closure was 1 month. Successfully closed fistulas had a mean duration of existence before plug placement of 4.6 months. Failed closures had a mean duration of existence before plug placement of 15.3 months. One of the five failed closures had a recurrence at 8 months. Fistulae originating from a percutaneous enteric tube, either a gastrostomy, ileostomy, or colojejunostomy, had success in two of three patients (66%). Fistulae due to diverticulitis did not achieve closure (0 of 3).
DISCUSSION
The enteric fistula patient population is one left with few treatment options. The literature on this population includes non-operative treatments such as negative pressure wound therapy, endoscopic clips, lasers, gelatin sponge, bioadhesives, and octreotide infusion, with reported fistula closure rates of 60–100%.[4,8,9,10,19] Extracellular matrix fistula plugs have been use in the literature for closure of anal fistulae, with success rates of 24–80%.[11-14] Prior studies and this one show even more encouraging data than the manufacturer’s quoted success rate of complete fistula closure in 21% of patients and drainage reduction in 21% of patients.[20] However, there have been few studies that have continued to look at ECMFPs for fistulae refractory to surgical closure or other closure methods with careful patient selection and longer follow-up.
The results in this series of patients indicate that there is a patient population with few options for which ECMFPs are indicated and may provide successful fistula closure or symptom relief for an extended period. The most successful patients in this series had fistulae originating in the small bowel or stomach with a mean duration of existence before plug placement of 4.6 months. High closure rates were achieved in these patients, with 75% of ECF achieving closure. The results also suggest that patients with fistulae that have existed for longer than 1 year are poor candidates, as none of these patients achieved closure. Because the technical success of the plug was defined as closure with no later recurrence, none of the five successful patients had further leakage from the fistula site once the wound achieved closure. In addition, none of the successful patients had a recurrence of the fistula after 6 months. Finally, patients with fistulae etiologies related to gastrostomy, ileostomy, or jejunostomy achieved closure 66% of the time, which would indicate that patients with a similar etiology may have better success. Likewise, none of those patients with fistulae of origin related to diverticulitis achieved closure, which may indicate that patients of a similar etiology may have less success. There are a few reasons this may have been the case. Every fistula that occurred secondary to diverticulitis in this sample also had a multiplicity of side tracts, which may have hampered healing from a single plug in the main tract. In addition, these patients with diverticulitis all have a strong background of chronic inflammation which contributes to poor wound healing, especially in the setting of an ECMFP placement. Finally, the three fistulae secondary to diverticulitis also had associated abscess cavities which create a local environment hostile to uniform healing into the extracellular matrix. Other factors relating to wound healing in our patient population were a history of smoking, long-term steroid use, diabetes diagnosis, and previous chemotherapy and radiation treatment. Two patients with a history of smoking had successful fistula closure and four did not. Only two patients in this study were taking prescribed long-term corticosteroids. Of these two, one had successful closure and the other did not. Two patients in this study had a previous diagnosis type 2 diabetes mellitus and both patients achieved successful closure. Only two patients had a history of chemotherapy treatment, and one of these two achieved successful closure. One patient had prior radiation and did not achieve closure. This study did not find a correlation between procedural success, as defined as fistula closure at 6 months, and with length or diameter of the fistula tracts treated.
This study has several limitations. The sample size is small due to the small number of patients who were treated with ECMFPs during the study period. In addition, the study was retrospective with no comparable procedure and thus could fall prey to bias associated with single-arm retrospective studies. There are also several comorbidities or potentially confounding factors relating to healing that could not be controlled or accounted for, such as nutrition status or physical activity which may well have contributed to recurrence or plug failure.
CONCLUSION
Extracellular matrix fistula plugs are a viable option to consider in a patient population that has few other treatments. They can be useful for the closure of refractory fistulae, especially fistulae involving the stomach or small bowel with an existence of <1 year. Patients with fistulae of colonic origin, patients who have had fistulae for longer than 1 year, or patients whose fistulae are due to diverticulitis are less likely to see successful closure with the ECMFP. Further studies are certainly indicated to assess more completely the clinical utility, role, and safety of ECMFPs in treating fistulae.
Ethical approval
The research/study was approved by the Institutional Review Board at Northwestern Feinberg School of Medicine/Northwestern Memorial Hospital, number STU00086279, dated September 12, 2013.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Nutrition and management of enterocutaneous fistula. Br J Surg. 2006;93:1045-55.
- [CrossRef] [PubMed] [Google Scholar]
- Enterocutaneous fistula: Are treatments improving? Surgery. 2006;140:570-6. discussion 576-8
- [CrossRef] [PubMed] [Google Scholar]
- Current management of enterocutaneous fistula. J Gastrointest Surg. 2006;10:455-64.
- [CrossRef] [PubMed] [Google Scholar]
- Interventional radiologic management and treatment of enterocutaneous fistulae. J Vasc Interv Radiol. 2015;26:7-19.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical management of high output enterocutaneous fistulae: A 24-year experience. Curr Opin Clin Nutr Metab Care. 2004;7:309-16.
- [CrossRef] [PubMed] [Google Scholar]
- An 11-year experience of enterocutaneous fistula. Br J Surg. 2004;91:1646-51.
- [CrossRef] [PubMed] [Google Scholar]
- Management of enterocutaneous fistulas: 30-year clinical experience. Chin Med J (Engl). 2003;116:171-5.
- [Google Scholar]
- Treatment of enterocutaneous fistulas, then and now. Nutr Clin Pract. 2017;32:508-15.
- [CrossRef] [PubMed] [Google Scholar]
- Enterostomal therapy and wound care of the enterocutaneous fistula patient. Clin Colon Rectal Surg. 2010;23:161-8.
- [CrossRef] [PubMed] [Google Scholar]
- Plugs unplugged. Anal fistula plug: The Concord experience. ANZ J Surg. 2010;80:341-3.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of anal fistula plug vs. fibrin glue in closure of anorectal fistulas. Dis Colon Rectum. 2006;49:371-6.
- [CrossRef] [PubMed] [Google Scholar]
- Results of collagen plug occlusion of anal fistula: A multicentre study of 126 patients. Colorectal Dis. 2014;16:626-30.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of transsphincteric anal fistulas by endorectal advancement flap or collagen fistula plug: A comparative study. Dis Colon Rectum. 2009;52:18-22.
- [CrossRef] [PubMed] [Google Scholar]
- Anal fistula plug and fibrin glue versus conventional treatment in repair of complex anal fistulas. Am J Surg. 2009;197:604-8.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of foregut fistula with biologic plugs. Surg Endosc. 2015;29:2006-12.
- [CrossRef] [PubMed] [Google Scholar]
- First experience with the use of a collagen fistula plug to treat enterocutaneous fistulas. J Vasc Interv Radiol. 2013;24:1559-65.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous treatment of a duodenocutaneous high-flow fistula using a new biological plug. Diagn Interv Radiol. 2015;21:247-51.
- [CrossRef] [PubMed] [Google Scholar]
- Extracellular matrix enterocutaneous fistula plugs show promise for low-flow colocutaneous and enterocutaneous fistulae. J Vasc Interv Radiol. 2021;32:128-34.
- [CrossRef] [PubMed] [Google Scholar]
- Laser ablation facilitates closure of chronic enterocutaneous fistulae. J Vasc Interv Radiol. 2018;29:335-9.
- [CrossRef] [PubMed] [Google Scholar]
- Enterocutaneous fistula repair Bloomington: Cook Medical; 2011. Available from: https://www.cookmedical.com/wp-content/uploads/sites/8/2015/08/efpsur-bm-efpmp-en-201112-finaldraft.pdf [Last accessed on 2022 Jul 27]
- [Google Scholar]






