Translate this page into:
How We Do It: Sphenopalatine Ganglion Blockade for Migraine Treatment

-
Received: ,
Accepted: ,
How to cite this article: Forrest A, Cantos A, Butani D. How We Do It: Sphenopalatine Ganglion Blockade for Migraine Treatment. Am J Interv Radiol 2018, 2(14) 1-4.
Abstract
Migraine is a common disorder with a range of available abortive and prophylactic treatments. Sphenopalatine ganglion blockade is an effective and safe option for treatment and prevention of migraine disorders. We present an instructional article for sphenopalatine ganglion blockade, including recommended patient selection, treatment procedure, and clinical follow up.
Keywords
Migraine
Migraine treatment
SphenoCath
Sphenopalatine ganglion block
INTRODUCTION
Migraine is a common, often disabling neurologic disorder characterized by its pulsatile nature, unilateral location, and prolonged duration. It can be associated with nausea, vomiting, photophobia, or phonophobia and potentially with sensory (most commonly visual), verbal, or motor aura. Common triggers include emotional stress, hormonal changes, inconsistent meals, weather, and sleep disturbances. Migraine affects one out of seven Americans annually, with the highest prevalence in women aged 25–55.[1] The pathophysiology of migraine is complex, involving activation of the trigeminovascular system that leads to inflammatory changes in the pain-sensitive meninges and alters the permeability of the blood–brain barrier.[2] Given the complex neurobiology of this disorder, treatment of migraine is often difficult and refractory.
Current management of migraine includes abortive therapy for acute attacks and preventive therapy to reduce the frequency and severity of recurrent headaches. Abortive therapy includes nonsteroidal anti-inflammatory drugs (NSAIDs) and analgesics, with level A recommendations for acetylsalicylic acid, ibuprofen, naproxen, diclofenac, and acetaminophen.[1] These therapies may also be combined with caffeine. Many migraine-specific therapies target the 5-HT serotonin receptors, as agonists at these receptors result in intracranial extra-cerebral vasoconstriction and inhibition of peripheral and central trigeminal nociceptive terminals. Triptans are 5-HT1B/1D agonists that can be used in moderate-to-severe migraines and are effective in 60% of NSAID non-responders; however, sustained relief may not occur, with headache recurrence in 15–40% of patients.[1] The most common side effects of triptan use include paresthesia, flushing, tingling, and mild transient chest pressure. Triptan use is contraindicated in patients with myocardial infarction, stroke, peripheral vascular disease, or untreated vascular risk factors.[3]
A common complication of episodic acute migraine treatment is the development of a medication overuse headache (MOH). Development of MOH can be limited by reducing triptan use to fewer than 10 days per month.[1] Chronic migraine, defined as 15 or more headache days per month for more than 3 months, occurs in 1% of the United States and commonly occurs in the setting of MOH.
In patients with a chronic migraine or frequent and/or disabling episodic migraine, preventive treatment should be explored to improve quality of life and decrease disability. Preventive treatment for migraine has multiple aims, including decreasing attack frequency by 50%, decreasing headache intensity and duration, improving acute therapy responsiveness, improving overall function, and decreasing occurrence of MOH. The choice of preventive treatment includes consideration of patient comorbidities, drug interactions, and side effects.[1] In addition to non-pharmacologic methods such as stress management and relaxation techniques, commonly used medications for migraine prevention include beta-blockers and antiepileptics.[4] On average, 40–45% of patients taking prophylactic medications experience a 50% reduction in migraine frequency, possibly limited by adherence due to drug side effects.[3]
OnabotulinumtoxinA (BTA) is approved for the management of chronic migraine. This treatment is delivered as intramuscular injections targeting 31 different head and neck sites every 12 weeks. BTA blocks acetylcholine release at the synaptic cleft, resulting in the autonomic blockade. In a meta-analysis by Jackson et al., BTA was associated with fewer headaches per month and a greater likelihood of experiencing a 50% reduction among patients with chronic migraine headaches, compared to placebo.[5] Further, Hepp et al. showed that when compared to oral migraine prophylaxis, BTA was associated with a significantly lower likelihood of headache-related ED visits and hospitalizations at 6, 9, and 12 months after initiating treatment.[6]
Sphenopalatine ganglion (SPG) blockade, the focus of this instructional article, is another emerging therapy for patients with chronic migraine. Greenfield Sluder first described the SPG in association with facial pain syndromes in 1909, and it is now targeted in the treatment of multiple disorders including sphenopalatine neuralgia, atypical facial pain, migraine, cluster headache, and herpes zoster.[7]
The SPG is located in the pterygopalatine fossa and is one of the four cranial parasympathetic ganglia. Activation of parasympathetic synapses within the SPG releases vasoactive peptides that contribute to neurogenic inflammation, vasodilation, and the symptoms of migraine.[8] Cranial autonomic symptoms commonly associated with migraine, such as lacrimation, conjunctival injection, eyelid edema, nasal congestion, and facial swelling, may be mediated by this parasympathetic outflow of the SPG.[9] By applying topical local anesthetic to the SPG, parasympathetic and sensory outflow may be attenuated, thereby treating migraine and its associated autonomic symptoms.
Blockade of the SPG has been achieved through transnasal, transoral, and infrazygomatic arch application of local anesthetics. Today, there are three FDA approved devices exist for delivery of blocking agents, namely SphenoCath® (Dolor Technologies, Scottsdale, Arizona, USA), Allevio™ (Jet Medical, Schwenksville, PA, USA), and Tx360® (Tian Medical Inc., Lombard, IL, USA), all which approach the ganglion transnasally.[10]
The effectiveness of this treatment in acute and long-term relief of migraine has been shown. Cady et al. described SPG blockade in patients with a chronic migraine using the Tx360® device. In this setup, the device is advanced below the middle turbinate to the pterygopalatine fossa, where 0.3 cc of 0.5% bupivacaine is injected. In 26 patients treated with bupivacaine compared to 12 patients treated with saline, significant headache relief was noted at 15 and 30 min with sustained relief at 24 h.[11] In follow-up of the same patient group, decreased number of headache days at 1 month post-treatment, decreased acute medication usage at 6 months, and improved quality of life at 6 months were all reported.[12]
Similar effectiveness of SPG blockade was reported in a study by Mandato et al. They studied the response of 112 patients with a chronic headache who were treated with 4% of xylocaine using the Allevio™ device. At 30 days, 88% of patients required less medication for ongoing migraine relief, with a 36% point reduction in the visual analog scale score used to quantify the degree of debilitation.[13]
Preliminary evidence shows SPG blockade is an effective and safe option for treatment and prevention of migraine disorders. We will describe how the authors use the SphenoCath® device.
Clinical evaluation of the patient
All patients are seen in consult. A level III E and M visit is documented with migraine disability assessment (MIDAS) scores recorded. The nares and posterior oropharynx are visually inspected with an otoscope. Informed consent is obtained. We discuss transient hypotension, dysphagia with possible aspiration, and arranging for a driver for the treatment. Relative contraindications include pre-existing cardiac rhythm abnormalities and severe hypotension. Patient evaluation should include pre-existing medical conditions, current medications, and allergies. Baseline blood pressure and heart rate pre-procedure should also be obtained.
Indications/contraindications for the procedure
This procedure is indicated in patients with frequent, refractory episodic, or chronic migraine who have not achieved adequate migraine control with medical prophylaxis or who do not want or are not candidates for onabotulinumtoxinA injections. Contraindications include allergy to lidocaine, nasal canal atresia or stenosis, inability to thread the catheter, and unstable cardiac arrhythmia.
Equipment needed
SphenoCath® Device
Lidocaine (5 mL of 4%, lidocaine jelly)
Fluoroscopy
Procedural steps
Anesthetize nasal passageway before device insertion with a small quantity of lidocaine jelly on a Q-tip.
Place patient in supine position with cervical spine extension. The C arm is rotated into a cross-table lateral. The neck is extended, and the head is adjusted, so the mandibular angles are superimposed.
Insert SphenoCath® along the anterior nasal passage and place superior to the middle nasal turbinate (Figures 1 and 2).
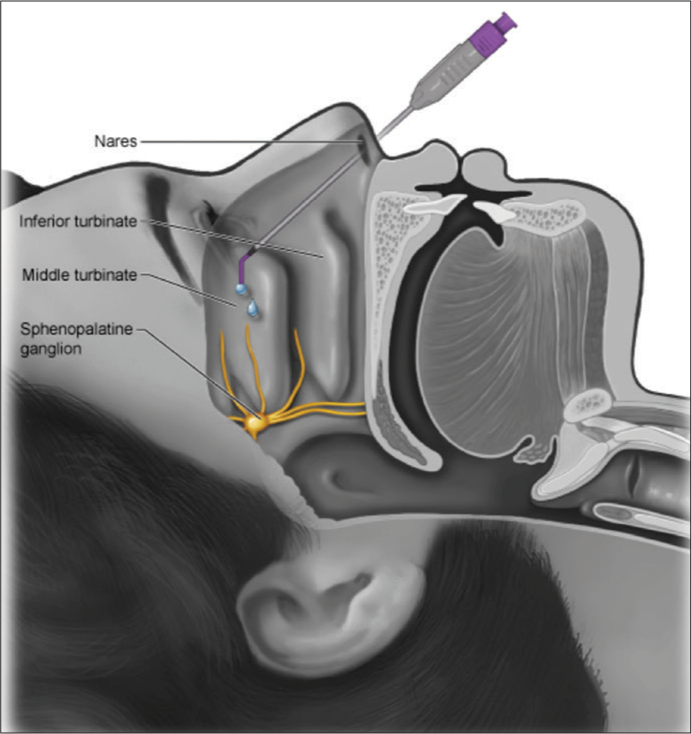 Figure 1
Figure 1- Technique of intranasal sphenopalatine ganglion block.
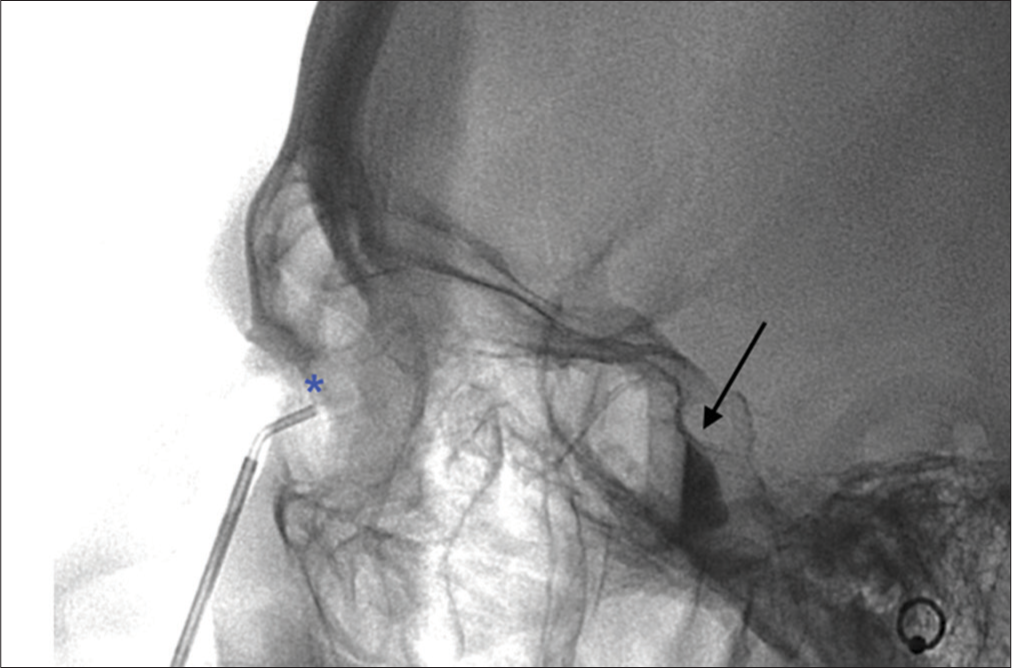 Figure 2
Figure 2- A 42-year-old female patient with chronic, refractory migraine, not relieved by medical therapy. Cross-table lateral fluoroscopic image shows the transnasal placement of the sphenocath device, with the tip deployed above the middle turbinate (*). Typical fluid-fluid level in the pterygopalatine fossa (arrow) is shown.
Use fluoroscopy to confirm the location of the tip of the sheath.
Advance inner catheter. Slowly inject 1–2 mL of contrast under fluoroscopy to visualize a fluid/fluid level (or transient delay of flowing contrast) in the pterygopalatine fossa.
Next, administer 2 mL of 4% lidocaine to saturate the PPF.
Remove device and repeat on the opposite side.
The patient is maintained in a supine position for 10 min.[10]
Adverse effects
Blood pressure and heart rate should be checked pre-procedure and post-procedure, as transient low blood pressure is a possible effect of treatment.
Patients may feel mild discomfort or burning during the procedure, and the medication may lead to an unpleasant taste.
There may be numbness in the back of the throat after the procedure. Patients should avoid eating or drinking until the numbness subsides to avoid choking.
Nausea and epistaxis may also occur.[10]
Clinical follow-up
Patients are instructed to contact the provider in case of adverse events.
Unless the patient experiences complete relief for 1 week, treatments are repeated weekly for a total of three treatments, to achieve stepwise decrease in symptoms. If the patient is satisfied with the level of relief after the three treatments, maintenance treatments can be performed every 6–8 weeks. The authors have patients who have experienced lasting relief up to 6 months with just a single treatment. This, however, is the exception rather than the rule.
Our practice defines clinical success as a minimum of 50% reduction in severity and/or frequency of symptoms, lasting for at least 30 days. Other indirect signs of success are improved MIDAS score, decreased use of medications, and decreased disability. Patients can choose to terminate the treatments at any time if they are not satisfied with the amount of symptom relief. Our success rate using the above criteria is 61%, well in line with other treatment modalities. SPG blockade can also be used as an adjunct with other treatment modalities.
CONCLUSIONS
Sphenopalatine ganglion blockade is a treatment option for those who suffer an episodic or chronic migraine, as it shown to be effective as acute migraine treatment and prevention of recurrent migraine. The SphenoCath® method described above is a safe and reproducible method to achieve SPG blockade. Research is currently being conducted to further characterize the effectiveness of this device in both acute and long-term migraine treatment.
Declaration of patient consent
Patient's consent not obtained as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol. 2015;14:1010-22.
- [CrossRef] [Google Scholar]
- A comparative effectiveness meta-analysis of drugs for the prophylaxis of migraine headache. PLoS One. 2015;10:e0130733.
- [CrossRef] [PubMed] [Google Scholar]
- Botulinum toxin A for prophylactic treatment of migraine and tension headaches in adults: A meta-analysis. JAMA. 2012;307:1736-45.
- [CrossRef] [PubMed] [Google Scholar]
- Comparative effectiveness of onabotulinumtoxinA versus oral migraine prophylactic medications on headache-related resource utilization in the management of chronic migraine: Retrospective analysis of a US-based insurance claims database. Cephalalgia. 2016;36:862-74.
- [CrossRef] [PubMed] [Google Scholar]
- Sphenopalatine ganglion blockade: A review and proposed modification of the transnasal technique. Pain Physician. 2004;7:283-6.
- [Google Scholar]
- Sphenopalatine ganglion block in the management of chronic headaches. Curr Pain Headache Rep. 2017;21:27.
- [CrossRef] [Google Scholar]
- Sphenopalatine ganglion neuromodulation in migraine: What is the rationale? Cephalalgia. 2014;34:382-91.
- [CrossRef] [PubMed] [Google Scholar]
- The sphenopalatine ganglion: Anatomy, pathophysiology, and therapeutic targeting in headache. Headache. 2016;56:240-58.
- [CrossRef] [PubMed] [Google Scholar]
- A double-blind, placebo-controlled study of repetitive transnasal sphenopalatine ganglion blockade with tx360(®) as acute treatment for chronic migraine. Headache. 2015;55:101-16.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term efficacy of a double-blind, placebo-controlled, randomized study for repetitive sphenopalatine blockade with bupivacaine vs. Saline with the tx360 device for treatment of chronic migraine. Headache. 2015;55:529-42.
- [CrossRef] [PubMed] [Google Scholar]
- Image-guided sphenopalatine ganglion blocks: An IR solution for chronic headaches. J Vasc Interv Radiol. 2015;26:S40.
- [CrossRef] [Google Scholar]






