Translate this page into:
Comparison of radiologic response criteria as predictors for refractoriness and survival to transarterial chemoembolization among hepatocellular cancer: Outcomes from a Southeast Asian cohort

*Corresponding author: Rudolf V. Kuhn, Department of Medical Imaging and Therapeutic Radiology, National Kidney and Transplant Institute, Quezon City, Philippines. rudolfvkuhn@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Kuhn RV, Ignacio GM, Jamias JD. Comparison of radiologic response criteria as predictors for refractoriness and survival to transarterial chemoembolization among hepatocellular cancer: Outcomes from a Southeast Asian cohort. Am J Interv Radiol 2022;6:3.
Abstract
Objectives:
Radiologic response helps select patients with hepatocellular carcinoma who may become refractory after repeated sessions of transarterial chemoembolization (TACE). The utility of the various criteria in assessing radiologic response and survival is, however, poorly defined. This study aimed to compare the modified response evaluation criteria in solid tumors and Choi criteria as well as identify other predictors of overall survival and refractory disease of HCC patients undergoing repetitive TACE.
Material and Methods:
The radiologic response, as well as clinical and laboratory characteristics of 39 patients treated with repetitive conventional TACE from January 2012 to January 2019, were analyzed in a retrospective cohort.
Results:
The median overall survival of patients was 23.2 months and overall mortality was 36%. Multivariate Cox regression analysis revealed that progressive disease (PD) using Choi criteria (HR = 5.47, CI 1.15–25.99, P = 0.033) and enhancement on follow-up CT (computed tomography) imaging (HR = 1.98, P = 0.034) were independent risk factors for poor survival as were Child-Pugh score (Hazard ratio = 3.47, P = 0.044), AST (HR = 7.6, P = 0.021), tumor size (HR = 5.47, P = 0.033), and neutrophil-lymphocyte ratio (HR = 1.25, P = 0.049). Multivariate analysis also showed that ALT (P = 0.005), enhancement (P = 0.003), Child-Pugh score (P = 0.010), and PD using Choi criteria (P = 0.022) were predictive of TACE refractoriness/failure.
Conclusion:
Predictors for poorer survival and TACE failure/refractory disease were identified. Radiologic response using the Choi criteria and persistent contrast enhancement on radiologic follow-up is ominous imaging signs on patient surveillance and should be included in a rational treatment strategy and the decision to switch therapy.
Keywords
Transarterial chemoembolization
Liver cancer
Response
Survival
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third leading cancer in the Philippines in both sexes, where its high incidence is largely attributed to the high endemicity of hepatitis B-related cirrhosis. It is the second leading cancer among males and ninth among females. In 2020, there were an estimated 10594 new HCC cases and 9953 deaths related to HCC.[1] Worldwide, HCC ranks as the fifth most common malignancy and the third leading cause of cancer-related deaths worldwide.[2] HCC is four to eight times more common in men and is usually associated with chronic liver injury, due to hepatitis B (HBV), hepatitis C (HCV), and alcoholic cirrhosis.[3]
Intermediate-stage HCC, as defined by the Barcelona clinic liver cancer (BCLC) staging system, is treated by transarterial chemoembolization (TACE) which is considered the treatment option of choice for intermediate-stage HCC. Meanwhile, the BCLC staging system also functions as an allocation system in many countries around the world, which links prognostic factors, stages, and treatment proposals.[4]
Conventional TACE consists of selective or even super-selective injection of a single or combination of chemotherapeutic agents emulsified in a viscous carrier (i.e., lipiodol). If liver function allows, this procedure is followed by injection of embolic material into the feeding arteries of the tumor. In this manner, TACE can obtain higher intratumoral drug concentrations compared with intravenous therapy, with additional tumor infarction and necrosis due to vascular occlusion. TACE may be repeated in patients with inadequate uptake of the chemoembolic agent and due to multiplicity of lesions.
Response evaluation criteria in solid tumors, version 1.1 (RECIST 1.1), is currently a standard method for the evaluation of tumor response in patients in clinical trials and is considered to be a surrogate endpoint to predict survival outcome in patients with solid tumors.[5] The apparent mismatch between the overall survival benefit of sorafenib in HCC and low objective response rate of < 5% by RECIST 1.1 has raised concerns as to the appropriateness of these criteria as a surrogate endpoint for survival in HCC. Ideally, response criteria should assess residual viability indicating the need for additional treatment. Various other criteria have therefore been proposed taking into account viability, such as the Choi and modified response evaluation criteria in solid tumors (mRECIST) criteria, but their efficacy as a predictor for survival in repetitive TACE sessions has not been assessed.
This study aimed to compare the efficacy of two widely used radiologic response criteria, namely Choi and mRECIST, in predicting overall survival and TACE refractory disease/ failure. Prediction of the refractory state to TACE early in the course of therapy would enable earlier changes in treatment strategy, such as combination therapy with molecular-targeted agents or switching to other therapeutic modalities.
Objectives
General objectives
The objective of the study was to compare the efficacy of Choi and mRECIST radiologic response criteria as predictors for overall survival and TACE refractoriness among HCC patients who underwent repeated sessions of TACE at a tertiary referral center in the Philippines.
Specific objectives
In detail, this study aims to accomplish the following:
Describe the baseline demographic, clinical, and radiologic characteristics of HCC patients who underwent TACE
Determine the proportion of HCC patients evaluated to have stage progression according to these criteria, predictive of TACE refractoriness.
Determine the overall survival of patients who underwent repeated TACE.
MATERIAL AND METHODS
Study design
Retrospective Cohort analysis.
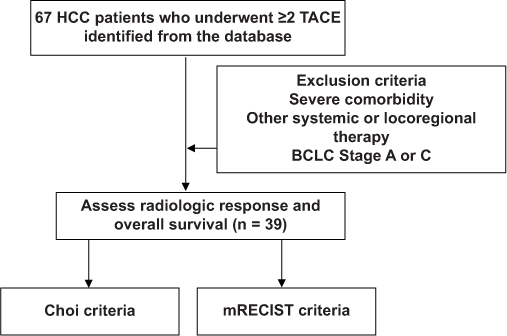
Study subjects and inclusion criteria
A total of 67 records of HCC patients above 18 years of age staged as BCLC B who underwent TACE from January 2012 to January 2019 were retrieved from the Medical Records Section of the hospital, with the picture archiving and communication system having been introduced in our institution in 2012. The diagnosis of HCC was based on histological examination or enhancement pattern consistent with HCC on dynamic liver imaging, such as computed tomography or magnetic resonance imaging, in conjunction with elevated level of serum a-fetoprotein, according to the criteria set by the European Association for the Study of the Liver (EASL) and were then classified as intermediate-stage HCC according to the BCLC criteria. A total of 39 patients were included who have received at least two TACE sessions. After each TACE session, a repeat CT/MR imaging must have been done within 12 weeks to assess tumor response as well as liver function parameters including the Child-Pugh Score. The first imaging follow-up was used as the basis for the radiologic imaging response criteria assessment.
The minimum simple size is 32, computed using GPower 3.4, with a level of significance of 0.05 and a beta of 0.20.
Exclusion criteria
Patients with one or more of the following were not included in this study: (a) Severe comorbidity, (b) other oral/intravenous systemic or locoregional therapy, such as local ablation therapy, and (c) either staged as BCLC Stage A or C (n = 5). Loss to follow-up will be treated on an intention to treat basis.
Data collection
Clinical parameters, including age, sex, comorbidities, etiology of HCC, Eastern Cooperative Oncology Group Performance Status Score (ECOG), Child-Pugh Score, neutrophil-to-lymphocyte ratio (NLR), and alpha fetoprotein, were through retrospective chart review. Baseline imaging at least 1 week before TACE and all follow-up CT imaging were also retrieved.
Treatment scheme and evaluation of response criteria
The conventional type of TACE was performed according to the protocol of our institution by three experienced and board-certified interventional radiologists. Each of the radiologists had at least 15 years of experience. Briefly, the procedure entails transfemoral placement of an arterial catheter using the Seldinger technique. Angiogram was performed to assess for aberrant tumoral feeders and portal flow. All patients studied received Mitomycin C exclusively to ensure homogeneity of the treatment effect. Standard dosages of the chemotherapeutic agent (between 10 and 20 mg) and the use of gelatin sponge particles were determined for each patient based on tumor burden and underlying liver function.
Repeated sessions of TACE were considered if residual viable or newly developed tumors were identified after the previous TACE, and were performed on an “on-demand” basis depending on individual tumor response and remaining liver function. Target lesion response was assessed based on the mRECIST criteria and Choi response criteria retrospectively and did not influence treatment allocation. These criteria categorize the tumor as having complete response, partial response (PR), stable disease (SD), and progressive disease (PD) with the Choi response criteria also evaluating residual enhancement.[6,7] The mRECIST criteria focus on the measurement of the enhancing tumor component during the arterial phase of the CT examination. Non-enhancing components are not measured. Meanwhile, the Choi response criteria, initially formulated by Choi et al. in 2007 for gastrointestinal tumors, focus on the measurement of the entire tumor diameter and the average attenuation, expressed as the Hounsfield unit. The sub-classification of these criteria is presented in the Appendix.[5-7]
TACE failure/refractoriness and stage progression was classified according to the Liver Cancer Study Group of Japan 2014 criteria update.[8] Patients’ charts were followed up to their most recent admission and/or until the time of their death. Tumor sizes and contrast attenuation values (HU) will be individually re-measured and compared with their follow-up on a Siemens® Syngo Via workstation (Siemens AG, Erlangen, Germany), according to the standard protocol described in the mRECIST and the CHOI response criteria.
Statistical analysis
Patient demographics and clinical characteristics before the first TACE as well as baseline tumor characteristics were presented using descriptive statistics. All valid data from evaluable subjects were included in the analysis. Listwise deletion approach was used to exclude incomplete data sets from the analysis.
For univariate analysis, the Kaplan–Meier method with log-rank test was used to calculate survival and to determine significance. Variables with P < 0.20 in univariate analysis were entered into a stepwise Cox regression model (conditional backward selection).
Spearman rho was used to test if there is a significant correlation between the number of TACE sessions and the patient’s state of TACE refractoriness.
All statistical analyses were performed using IBM SPSS Statistics 22 (Armonk, NY, USA). Reported two-tailed P-values were considered significant at < 0.05 at a 95% confidence interval.
RESULTS
Patient characteristics
[Table 1] summarizes the baseline patient characteristics of a cohort of 39 patients. Most of the patients were men (81.5%) with a median age of 59 years. The majority of patients (51.3%) had a good Eastern Cooperative Oncology Group performance status of 1, while 46.2% of patients had ECOG 0. Comorbidity was present in almost all patients, with hypertension present in 14 of patients (35.9%) followed by diabetes (17.9%). Lesser comoborbities such as dyslipidemia and arthritis were classified as others. Hepatitis B infection was found to be the most common cause of the liver disease (56.4%). About 82% of patients had compensated liver disease, belonging to Child-Pugh Class A. All patients were treatment-naïve at the time of enrolment. Patients had between 2 and 5 TACE sessions (41% had 2 sessions, 28% had 3 sessions, and 21% had 4 sessions, while 10% had 5 sessions).
| Number | Percentage | |
|---|---|---|
| Sex | ||
| Male | 32 | 82.1 |
| Female | 7 | 17.9 |
| Age (median, range) | 59 (51–85) | |
| ECOG Functional Status | ||
| 0 | 18 | 46.2 |
| 1 | 20 | 51.3 |
| 2 | 1 | 2.5 |
| 3 | 0 | 0 |
| 4 | 0 | 0 |
| 5 | 0 | 0 |
| Comorbid conditions | ||
| None | 5 | 12.8 |
| Hypertensive | 14 | 35.9 |
| Diabetic | 7 | 17.9 |
| Hypertensive and diabetic | 6 | 15.4 |
| Diabetic and others | 2 | 5.2 |
| Hypertensive and others | 5 | 12.8 |
| Etiology | ||
| Hepatitis B | 22 | 56.4 |
| Hepatitis C | 1 | 2.5 |
| NASH | 9 | 23.1 |
| Alcoholic | 5 | 12.8 |
| Cryptogenic | 2 | 5.2 |
| Child Pugh Class (baseline) | ||
| A | 32 | 82.0 |
| B | 7 | 18.0 |
| C | 0 | 0 |
NASH: Non-alcoholic steatohepatitis
Baseline tumor characteristics areare summarized in [Table 2]. A slight majority (61.5 %) had a single tumor. Tumor size ranged from 3.8 to 16.0 cm in cumulative size for patients with multiple tumors, with 48.7% of patients having sizes larger than 7 cm. Most patients only had unilobar involvement (61.5 %), with most HCC tumors observed in the right lobe.
| Number | Percentage | |
|---|---|---|
| Tumor Number | ||
| Single | 24 | 61.5 |
| Multiple | 15 | 38.5 |
| Tumor Size | ||
| <5 cm | 9 | 23.1 |
| 5–7 cm | 11 | 28.2 |
| >7 cm | 19 | 48.7 |
| Lobar Involvement | ||
| Right | 20 | 51.2 |
| Left | 4 | 10.3 |
| Bilobar | 15 | 38.5 |
Overall survival and univariate analysis of prognostic factors
During the study period, 14 patients (36%) died. The median overall survival of patients was 23.2 months (range of 4–66 months). At univariate analysis, radiologic response using CHOI criteria (P = 0.011) was significantly associated with survival [Table 3]. Other factors that were significantly associated with survival were refractory status (P = 0.007), age (P = 0.088), Child Pugh score (P = 0.200), NLR (P = 0.154), AST (P = 0.004), AFP ratio 1.85 above baseline (P = 0.189), Tumor Size (P = 0.004), Enhancement on post-TACE CT scan (P = 0.036), and ART score (P = 0.068).
| Variables | n | OS (months) mean | 95% | CI | P-value (log-rank) |
|---|---|---|---|---|---|
| mRECIST criteria | |||||
| StableDisease | 34 | 54 | 47 | −61 | 0.249 |
| Partial Response | 4 | 50 | 24 | −76 | |
| Progressive Disease | 1 | 26 | 26 | −26 | |
| CHOI criteria | |||||
| StableDisease | 16 | 56 | 47 | −66 | 0.011* |
| Partial Response | 14 | 60 | 53 | −67 | |
| Progressive Disease | 9 | 35 | 17 | −53 |
Multivariate analysis
All potentially relevant covariates, including ECOG performance status, were placed in the multivariate Cox regression analysis. After one-at-a-time stepwise removal of the covariates with P > 0.05 until all significant regression coefficients remained. PD on radiologic follow-up using CHOI criteria (HR = 5.47, CI 1.15–25.99, P = 0.033) and significant enhancement on follow-up CT imaging (HR = 1.98, CI 1.05–3.72, P = 0.034) were independent risk factors for poor survival. Furthermore, other predictors for poor survival included high Child Pugh score (Hazard ratio [HR] = 3.47, CI 1.31–9.15, P = 0.044), elevated AST (HR = 7.6, CI 1.36–46.61, P = 0.021), tumor size greater than 7.2 cm (HR = 5.47, CI 1.15–25.99, P = 0.033), and elevated NLR (HR = 1.25, CI 1.19–9.86, P = 0.049). The other potentially predictive factors, inclusive ECOG performance status, were found to be non-contributive to the model.
DISCUSSION
Repetition of TACE increases tumor response and prolongs survival, but it is necessary to identify patients, who may not benefit from TACE any longer and should be shifted to Sorafenib treatment for improved outcomes in prognosis. This necessitates designing a study to identify which radiologic response criteria predict TACE refractoriness and survival of these patients. To the best of our knowledge, the current study is the first study in the Southeast Asian setting to explore different radiologic response criteria as predictive factors for survival and TACE refractoriness/failure in a country where most patients have primarily viral hepatitis-related HCC.
The most common cause of HCC was viral in almost 60 % of patients, composed mainly of hepatitis B. This compares to most studies done in Asia.[9,10] Survival of patients who underwent TACE also compared to the previous studies with a range of 23.1–26.0 months.[10,11] These data demonstrate the efficacy of TACE procedures as well as the success of the mitomycin monotherapy employed at our institution. Stringent patient selection, e.g. intermediate-stage HCC in the BCLC B category, from a single-center cohort with similar treatment schemes and follow-up schedules reduced bias and assured homogeneity of the subjects.
Patients with stable disease or partial response had longer overall survival compared to patients who had progressive disease using the CHOI and mRECIST criteria. This result is mirrored by earlier studies that suggest that Choi, EASL, and mRECIST criteria appear more appropriate than RECIST 1.1 to identify responders with long survival among advanced HCC patients benefiting from sorafenib.[5] In specific, the Choi criteria emerged as a predictor for survival and TACE refractoriness in the present study and were superior to the mRECIST criteria. This correlates with the earlier findings that tumor response according to Choi criteria may be helpful to define early HCC patients who benefit from transarterial radioembolization.[7] In the aforementioned study, Choi criteria successfully identified patients with a long time to disease progression or short time to progression. Whereas, neither RECIST nor modified Choi criteria discriminated between patients who had a short or long clinical benefit.
The current study also showed that aside from radiologic response criteria, other clinical, and laboratory parameters should be taken into account when assessing a patient’s overall response to the treatment. Namely, high Child-Pugh score, elevated AST, tumor size, PD on radiologic follow-up, elevated NLR and significant enhancement on follow-up CT imaging were independent risk factors for poor survival. This is comparable to a previous Korean study where tumor size, AFP ratio, AST >95 U/L, AST increase >25%, and poor radiologic tumoral response emerged as significant predictors for poor prognosis.[9]
Identification of these clinical and laboratory variables allows the formulation of the tailored treatment protocol, rationalizing the switch to systemic chemotherapy early on.[12-14] This study also demonstrated the value of close interval radiologic follow-up using multiphasic imaging and the Choi criteria. Radiologists involved in the follow-up imaging of HCC patients should emphasize the degree of residual enhancement using the Hounsfield unit and areas of nodular enhancement to allow prognostication.
CONCLUSION
In summary, progressive disease in the Choi criteria and significant enhancement on follow-up CT imaging as independent risk factors for poor survival of patients. Other clinical and laboratory parameters such as liver function tests were also considered. Radiologic follow-up should emphasize the enhancement and adhere to the Choi criteria. A rational treatment strategy should be individualized considering the patient’s clinical condition, liver function, and response on radiologic follow-up and local factors, as highlighted in this study. A follow-up study considering also technical factors encountered during TACE is recommended for a more comprehensive analysis of survival of HCC patients.
Acknowledgments
The authors acknowledge the assistance of Dr. Catherine Teh, Dr. Rizza Antoinette So, and Dr. Justin Syling for their participation in an earlier stage of this research series. The authors also acknowledge Dr. Nikki Heherson Dagamac and Dr. Lisa Angelica Kuhn for their helpful criticism in the preparation of the manuscript. Preliminary findings of this research were presented at the 2021 annual congress of the Cardiovascular and Interventional Radiology Society of Europe.
Ethical approval
All patients’ data included in this study was for the use of the investigator only and was made strictly confidential. Anonymity was ensured using a randomly assigned patient identifier code in the data collected. The key for the patient identifier code was only being known to the investigator. The study strictly adhered to local and international guidelines, such as but not limited to the Good Clinical Practice Guidelines, the Helsinki Declaration, and International Ethical Guidelines for Biomedical Research Involving Human Subjects. No vulnerable populations were included in this study. No conflict of interest exists or has been identified.
This study was duly approved by the Research Ethics Committee review board of our hospital.
Declaration of patient consent
Institutional Review Board (IRB) permission was obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Cancer Incidence and Mortality Worldwide: IARC Cancer Base. 2020. Lyon, France: International Agency for Research on Cancer; Available from: http://globocan.iarc.fr
- [Google Scholar]
- Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis and follow-up. Ann Oncol. 2010;21(Suppl 5):59-65.
- [CrossRef] [PubMed] [Google Scholar]
- How to define transarterial chemoembolization failure or refractoriness: A European perspective. Liver Cancer. 2014;3:119-24.
- [CrossRef] [PubMed] [Google Scholar]
- Alternative response criteria (Choi, European association for the study of the liver, and modified response evaluation criteria in Solid Tumors [RECIST]) versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with Sorafenib. Oncologist. 2014;19:392-402.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging for assessment of treatment response in hepatocellular carcinoma: Current update. Indian J Radiol Imaging. 2015;25:121-8.
- [CrossRef] [PubMed] [Google Scholar]
- Choi criteria are superior in evaluating tumor response in patients treated with transarterial radioembolization for hepatocellular carcinoma. Oncol Lett. 2013;6:1707-12.
- [CrossRef] [PubMed] [Google Scholar]
- "Transarterial chemoembolization failure/ refractoriness: JSH-LCSGJ criteria 2014 update. Oncology. 2014;87(Suppl 1):22-31.
- [CrossRef] [PubMed] [Google Scholar]
- Severity and timing of progression predict refractoriness to transarterial chemoembolization in hepatocellular carcinoma. J Gastroenterol Hepatol. 2012;27:1051-6.
- [CrossRef] [PubMed] [Google Scholar]
- The effectiveness of ART score in selecting patients for transarterial chemoembolization retreatment. Medicine. 2015;94:1-7.
- [CrossRef] [PubMed] [Google Scholar]
- The ART score is not effective to select patients for transarterial chemoembolization retreatment in an Italian series. Digest Dis. 2014;32:711-6.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of sorafenib in intermediate-stage hepatocellular carcinoma patients refractory to transarterial chemoembolization. Oncology. 2014;87:330-41.
- [CrossRef] [PubMed] [Google Scholar]
- The ART of decision making: Retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology. 2013;57:2261-72.
- [CrossRef] [PubMed] [Google Scholar]
- Derived neutrophil to lymphocyte ratio predicts prognosis for patients with HBV-associated hepatocellular carcinoma following transarterial chemoembolization. Oncol Lett. 2016;11:2087-994.
- [CrossRef] [PubMed] [Google Scholar]
APPENDIX
| EASL | mRECIST | Choi Criteria |
|---|---|---|
| CR | ||
| Disappearance of intratumoral arterial enhancement | Disappearance of all lesions and pathologic lymph nodes | Disappearance of all lesions |
| PR | ||
| ≥50% decrease in sum of the arterial enhancing areas | ≥ 30% decrease in the sum of diameters of enhancing target lesions | ≥10% decrease in longest diameter of target lesion or≥15% decrease in attenuation (HU) |
| SD | ||
| Neither PR nor PD | Neither PR nor PD | Neither PR nor PD |
| PD | ||
| ≥25% increase in size of the arterial enhancing areas or development of new lesion | ≥20% increase in sum of diameters of viable target lesions recorded since treatment started or development of new lesions | ≥10% increase in the longest diameter of target lesions without PR criteria or development new lesions |
CR: Complete response, PR: Partial response, SD: Stabledisease, EASL: European Association for the Study of the Liver, mRECIST: Modified response evaluation criteria in solid tumors







