Translate this page into:
Retrospective evaluation of image-guided cholecystostomy tube utilization and outcomes during the first wave of the COVID-19 pandemic

-
Received: ,
Accepted: ,
How to cite this article: Snyder A, Salamone S, Reid NJ, Yeung T, Di Capua J, Som A, et al. Retrospective evaluation of image-guided cholecystostomy tube utilization and outcomes during the first wave of the COVID-19 pandemic. Am J Interv Radiol 2021;5:13.
Abstract
Objectives:
During the COVID-19 pandemic, there was a perceived increase in the number of cholecystostomy tube placements. We have retrospectively analyzed the incidence and outcomes of cholecystostomy tube placement during the COVID-19 pandemic surge.
Material and Methods:
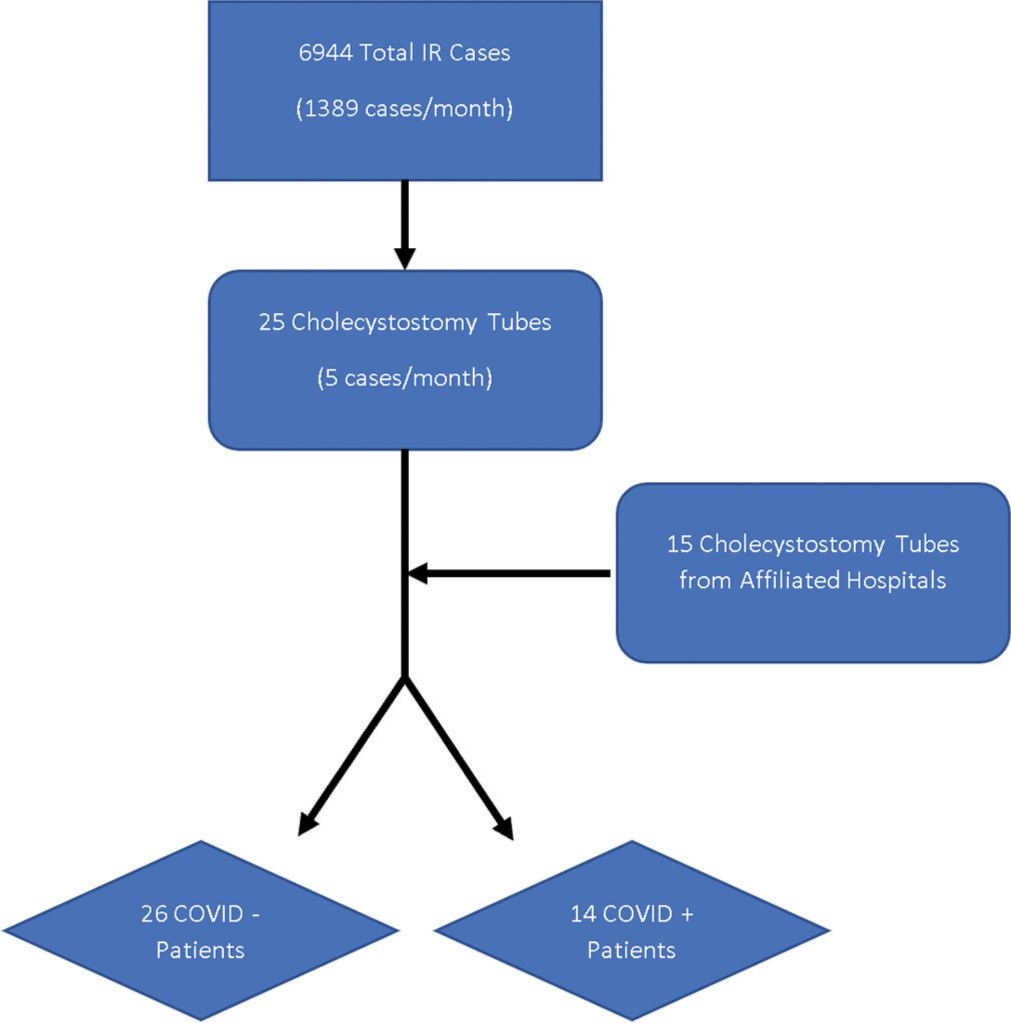
Cholecystostomy tube placement and overall interventional radiology (IR) case volume were analyzed at our tertiary care center during the pandemic (March 15, 2020–July 30, 2020) and compared to the same time period in 2019. In addition, an age- and gender-matched control study of outcomes for 40 patients (25 from our home institution and 15 from our affiliated hospitals) grouped by COVID-19 status who received percutaneous cholecystostomy tubes between March 15, 2020, and July 30, 2020, was performed.
Results:
We observed a significant increase in relative cholecystostomy tube volume during the pandemic, despite a decrease in total IR case volume. There was no significant difference in pre- or post-procedural laboratory data, vital signs, imaging, or mortality between COVID-positive and COVID-negative patients who received cholecystostomy tubes.
Conclusion:
Percutaneous cholecystostomy tube placement is likely a safe treatment for acalculous cholecystitis in patients with COVID-19 with equivalent outcomes to patients without COVID-19.
Keywords
Cholecystitis
Cholecystostomy tube
COVID-19
Pandemic
INTRODUCTION
During the current COVID-19 pandemic, there has been an observed increase in referrals to the interventional radiology (IR) department at our tertiary care hospital for percutaneous cholecystostomy tube placement.
Two potential driving factors leading to the increase in percutaneous cholecystostomy tube requests during the COVID-19 pandemic include (1) increased incidence of severely ill patients in the ICU receiving nothing by mouth with fevers, elevated white blood cell (WBC) counts, and ultrasound examinations showing distended gallbladders and (2) increasing pressure on ICU physicians who are battling a novel, severe illness, to pursue any avenue to potentially improve the prognosis of their patients. These observations have similarly been expressed by interventional radiologists practicing in areas that were severely affected by the COVID-19 pandemic.[1]
The purpose of this study is 2-fold: (1) To analyze the incidence of cholecystostomy tube placement during the first wave of the COVID-19 pandemic compared to 2019 and (2) to determine the differences in outcomes of cholecystostomy tube placement between COVID-positive and COVID-negative patients.
MATERIAL AND METHODS
This is a retrospective review of the incidence and outcomes of cholecystostomy tube cases referred to an IR department at a tertiary care hospital located in an epicenter during the first wave of the COVID-19 pandemic defined as March 15, 2020–July 30, 2020. For a comparative incidence of percutaneous cholecystostomy tube placement, reference was made to a similar time period 1 year prior, March 15, 2019– July 30, 2019.
After comparing the incidence of percutaneous cholecystostomy tube placement between 2019 and 2020, we analyzed 40 patients (25 from our home institution and 15 from our affiliated hospitals) during the pandemic from March 15, 2020, to July 30, 2020. These 40 patients were grouped based on COVID-19 infection status (14 COVID positive and 26 COVID negative) then matched by age and sex to their COVID-19-negative counterparts [Figure 1]. Analyzed data included patient demographics, admission symptomatology, comorbidities, pre-procedure imaging, pre- and post-procedure laboratory data, and post-procedure outcomes which were retrospectively reviewed starting on September 1, 2020. Two-sided t-tests were performed for continuous variables and Chi-squared tests were performed for categorical variables. The set significance for all statistical tests was P < 0.05.
- Total IR case volume and cholecystostomy tube volume during the first wave of the COVID-19 pandemic defined as March 15, 2020–July 30, 2020. IR: Interventional radiology.
RESULTS
Cholecystostomy case data
From March 15, 2019, to July 30, 2019, an average of 2103 IR cases/month was performed, compared to an average of 1389 IR cases/month during the first wave of the COVID-19 pandemic in 2020 [Figure 2]. Of the total IR cases, an average of 5.8 cholecystostomy tube cases/month was performed during 2019 compared to 5 cases/month in 2020 [Figure 3]. Cholecystostomy tube cases comprised 0.28% of total IR cases in 2019, compared with 0.36% of total IR cases in 2020, P < 0.00001 [Figure 3].

- Distribution of IR case volume during the first wave of the COVID-19 pandemic defined as March 15, 2020–July 30, 2020, and pre-pandemic reference period defined as March 15, 2019–July 30, 2019. *The percentage of cholecystostomy tubes was calculated by dividing monthly cholecystostomy tube volume by monthly total IR case volume. IR: Interventional radiology.

- Distribution of cholecystostomy tube volume during the first wave of the COVID-19 pandemic defined as March 15, 2020–July 30, 2020 and pre-pandemic reference period defined as March 15, 2019–July 30, 2019. *The percentage of cholecystostomy tubes was calculated by dividing monthly cholecystostomy tube volume by monthly total IR case volume. IR: Interventional radiology.
Patient demographics
Forty patients (25 from our home institution and 15 from our affiliated hospitals) were analyzed during the study, 14 (35%) of which were COVID positive. Twenty-five (62.5%) were male and 15 (37.5%) were female. Of the 14 COVID-positive cases, 3 (21.4%) were female, compared to 12 (46.15%) females in the COVID-negative group, P = 0.13. The average age of COVID-positive patients was 66.1 years old (range 34.6–91.0, SD 17.7) compared to 60.1 years old (range 28.5–93.7, SD 26.2) for COVID-negative patients, P = 0.46 [Table 1].
| COVID negative | COVID positive | P-value | |||||
|---|---|---|---|---|---|---|---|
| % | SD | n | % | SD | n | ||
| Demographics | |||||||
| Age (mean) | (60.13) | 26.23 | 26 | (66.05) | 17.72 | 14 | 0.46 |
| Female | 46.15 | - | 12 | 21.43 | - | 3 | 0.13 |
| Admission symptoms | |||||||
| Fatigue | 57.69 | - | 15 | 50.00 | - | 7 | 0.65 |
| Nausea | 42.30 | - | 11 | 28.57 | - | 4 | 0.40 |
| Vomiting | 30.79 | - | 8 | 28.57 | - | 4 | 0.89 |
| Abdominal pain | 65.38 | - | 17 | 57.14 | - | 8 | 0.61 |
| Right upper quadrant pain | 50.00 | - | 13 | 50.00 | - | 7 | 1.00 |
| Chills | 23.08 | - | 6 | 7.14 | - | 1 | 0.21 |
| Cough | 26.92 | - | 7 | 57.14 | - | 8 | 0.06 |
| Shortness of breath | 34.62 | - | 9 | 50.00 | - | 7 | 0.35 |
| Diarrhea | 26.92 | - | 7 | 14.29 | - | 2 | 0.37 |
| Murphy’s sign | 15.38 | - | 4 | 21.43 | - | 3 | 0.64 |
| Comorbidities | |||||||
| Congestive heart failure | 26.92 | - | 7 | 21.43 | - | 3 | 0.71 |
| Arrhythmia | 38.46 | - | 10 | 28.57 | - | 4 | 0.54 |
| Diabetes mellitus | 30.79 | - | 8 | 57.14 | - | 8 | 0.11 |
| Malignancy | 38.46 | - | 10 | 14.29 | - | 2 | 0.11 |
| COPD* | 11.54 | - | 3 | 0.00 | - | 0 | 0.20 |
| Obesity | 15.38 | - | 4 | 28.57 | - | 4 | 0.33 |
| Chronic kidney disease | 19.23 | - | 5 | 28.57 | - | 4 | 0.51 |
| Liver disease | 7.69 | - | 2 | 0.00 | - | 0 | 0.30 |
| Hypertension | 69.23 | - | 18 | 71.43 | - | 10 | 0.89 |
| Peripheral vascular disease | 3.84 | - | 1 | 0.00 | - | 0 | 0.47 |
Patient comorbidities
The most common pre-procedural comorbidity overall was hypertension (28, 70%). The most common comorbidities in COVID-positive patients were hypertension (10, 71.4%), diabetes mellitus (8, 57.1%), obesity (4, 28.6%), and chronic kidney disease (4, 28.6%), while the most common comorbidities in COVID-negative patients were hypertension (18, 69.2%), arrhythmias (10, 38.5%), malignancy (10, 38.5%), and diabetes mellitus (8, 30.8%). Overall, there were no statistically significant differences in comorbidities between COVID-positive and COVID-negative patients [Table 1].
Pre-procedure clinical variables
The most common admission symptoms in COVID-positive patients were abdominal pain (8, 57.1%), cough (8, 57.1%), shortness of breath (7, 50.0%), and fatigue (7, 50.0%). The most common admission symptoms in COVID-negative patients were abdominal pain (17, 65.4%), fatigue (15, 57.7%), and nausea (42.3%). Eight (57.1%) COVID-positive and 7 (27.0%) COVID-negative patients reported a cough, P = 0.06. Three (21.4%) COVID-positive and 4 (15.4%) COVID-negative patients had a positive Murphy’s sign, although this was unable to be assessed in 7 (50%) COVID-positive patients that were intubated for respiratory failure before evaluation, P = 0.64. There were no statistically significant differences in pre-procedure clinical characteristics between COVID-positive and COVID-negative patients [Table 1].
Pre-procedure laboratories and vital signs
Several laboratory values and vital signs were evaluated within 24 h pre-procedure. Five (35.7%) COVID-positive and 11 (42.3%) COVID-negative patients had a fever within 24 h before their procedure, P = 0.70. The mean pre-procedure systolic blood pressure in COVID-positive patients was 121.7 ± 13.4 (SD) compared to 118.2 ± 19.3 (SD) for COVID-negative patients, P = 0.55. The mean WBC count for COVID-positive patients was 16.2 ± 6.7 (SD) compared to 13.01 ± 6.7 (SD) for COVID-negative patients, P = 0.17. The mean absolute neutrophil count was 13.1 ± 6.3 (SD) for COVID-positive patients compared with 10.9 ± 6.7 for COVID-negative patients, P = 0.33. The mean international normalized ratio (INR) was 1.3 ± 0.4 (SD) for COVID-positive patients compared with 1.6 ± 0.6 (SD) for COVID-negative patients, P = 0.17. There were no statistically significant differences in any of the pre-procedure laboratory values or vital signs analyzed between COVID-positive and COVID-negative patients [Table 2].
| COVID negative | COVID positive | P-value | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | n(%) | Mean | SD | n(%) | ||
| Laboratory values | |||||||
| White blood cell count | 13.01 | 6.74 | 26 | 16.17 | 6.73 | 14 | 0.17 |
| Red blood cell count | 3.66 | 0.87 | 26 | 3.63 | 0.58 | 14 | 0.91 |
| Hemoglobin | 10.73 | 2.47 | 26 | 10.23 | 2.00 | 14 | 0.52 |
| INR* | 1.57 | 0.57 | 24 | 1.32 | 0.40 | 13 | 0.17 |
| % neutrophils | 76.63 | 13.77 | 23 | 80.99 | 9.69 | 14 | 0.31 |
| Absolute neutrophil count | 10.89 | 6.7 | 23 | 13.09 | 6.28 | 14 | 0.33 |
| Alkaline phosphatase | 235.65 | 175.34 | 26 | 227.62 | 300.45 | 14 | 0.92 |
| Total bilirubin | 1.8 | 2.13 | 26 | 2.02 | 2.98 | 14 | 0.79 |
| Aspartate transaminase | 208.96 | 426.89 | 26 | 122.14 | 179.92 | 14 | 0.47 |
| Alanine transaminase | 106.77 | 136.15 | 26 | 87.43 | 103.96 | 14 | 0.65 |
| Vital signs | |||||||
| Fever | - | - | 11 (42.30) | - | - | 5 (35.71) | 0.70 |
| Systolic blood pressure | 118.19 | 19.34 | 26 | 121.69 | 13.37 | 14 | 0.55 |
| Diastolic blood pressure | 65.26 | 11.82 | 26 | 62.85 | 8.30 | 14 | 0.50 |
| Pre-procedure imaging | |||||||
| Distended gallbladder | - | - | 12 (48.00) | - | - | 8 (57.14) | 0.59 |
| Wall thickening | - | - | 20 (80.00) | - | - | 7 (50.00) | 0.05 |
| Pericholecystic fluid | - | - | 15 (60.00) | - | - | 5 (35.71) | 0.15 |
| Procedure location | |||||||
| Interventional radiology suite | - | - | 22 (84.60) | - | - | 10 (71.40) | 0.33 |
| Pre-procedure ICU admission | - | - | 10 (38.46) | - | - | 8 (57.14) | 0.26 |
| Pre-procedure intubation | - | - | 4 (15.40) | - | - | 7 (50.00) | 0.02 |
Pre-procedure imaging
Of the 40 patients, pre-procedure imaging to identify cholecystitis was performed with ultrasound (27, 67.5%) or CT (13, 32.5%). In COVID-positive patients, 8 (57.1%) patients had imaging findings of gallbladder distension, 7 (50%) patients had wall thickening, and 5 (35.7%) patients had pericholecystic fluid. In COVID-negative patients, 12 (48.0%) patients had gallbladder distension, 20 (80.0%) patients had wall thickening, and 15 (60.0%) patients had pericholecystic fluid. There was no statistically significant difference between COVID-positive and COVID-negative patients for any of the three imaging findings [Table 2].
Procedure location and ICU/intubation status
Cholecystostomy tubes were either placed in the IR suite (32, 80%) or at the patient’s bedside (8, 20%). Among COVID-positive patients, 10 (71.4%) cases were performed in the IR suite compared to 22 (84.6%) of COVID-negative patients, P = 0.33. The number of patients admitted to the ICU before their procedure was 8 (57.1%) for COVID-positive patients and 10 (38.5%) for COVID-negative patients, P = 0.26. The number of patients requiring pre-procedural intubation was 7 (50.0%) for COVID-positive patients and 4 (15.4%) for COVID-negative patients, P = 0.02 [Table 2].
Post-procedure laboratories, vital signs, and outcomes
Several laboratory values and vital signs were analyzed within 24 h after the procedure. Fever was present in 2 (14.3%) COVID-positive and 4 (15.4%) COVID-negative patients within 24 h after the procedure, P = 0.93. Mean post-procedure systolic blood pressure in COVID-positive patients was 123.4 ± 20.2 (SD) compared to 122.5 ± 18.0 (SD) for COVID-negative patients, P = 0.88 [Table 3].
| COVID negative | COVID positive | P-value | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | n(%) | Mean | SD | n(%) | ||
| Laboratory values | |||||||
| White blood cell count | 13.67 | 7.16 | 23 | 15.39 | 6.45 | 13 | 0.48 |
| Red blood cell count | 3.29 | 0.68 | 23 | 3.33 | 0.47 | 13 | 0.85 |
| Hemoglobin | 9.63 | 1.85 | 23 | 9.38 | 1.68 | 13 | 0.69 |
| INR | 1.67 | 0.45 | 13 | 1.32 | 0.22 | 13 | 0.02 |
| % neutrophils | 80.85 | 8.08 | 21 | 78.62 | 9.45 | 13 | 0.47 |
| Absolute neutrophil count | 11.77 | 7.30 | 21 | 11.98 | 5.55 | 12 | 0.93 |
| Alkaline phosphatase | 229.73 | 167.22 | 22 | 284.49 | 365.94 | 13 | 0.55 |
| Total bilirubin | 2.28 | 3.13 | 22 | 2.13 | 3.46 | 13 | 0.90 |
| Aspartate transaminase | 168.23 | 344.28 | 22 | 86.85 | 71.52 | 13 | 0.41 |
| Alanine transaminase | 93.64 | 106.32 | 22 | 77.46 | 61.11 | 13 | 0.62 |
| Vital signs | |||||||
| Fever | - | - | 4 (15.38) | - | - | 2 (14.29) | 0.93 |
| Systolic blood pressure | 122.52 | 17.98 | 26 | 123.43 | 20.19 | 14 | 0.88 |
| Diastolic blood pressure | 65.33 | 10.80 | 26 | 64.07 | 10.57 | 14 | 0.64 |
| Clinical outcomes | |||||||
| Discharged | - | - | 24 (92.31) | - | - | 10 (71.43) | 0.08 |
| Procedural complications | - | - | 4 (15.38) | - | - | 2 (14.29) | 0.93 |
| Death | - | - | 6 (23.08) | - | - | 5 (35.71) | 0.40 |
| Positive cholecystostomy tube microbiology | - | - | 14 (53.84) | - | - | 7 (50.00) | 0.82 |
| Antibiotic use | - | - | 25 (96.15) | - | - | 14 (100) | 0.46 |
The mean WBC count for COVID-positive patients was 15.4 ± 6.5 (SD) compared to 13.7 ± 7.2 (SD) for COVID-negative patients, P = 0.48. The mean absolute neutrophil count was 12.0 ± 5.6 (SD) for COVID-positive patients compared to 11.8 ± 7.3 for COVID-negative patients, P = 0.93. Positive cholecystostomy tube microbiology cultures were present in 7 (50%) COVID-positive patients and 14 (54%) COVID-negative patients, P = 0.82. The mean INR for COVID-positive patients was 1.32 ± 0.22 (SD) compared to 1.67 ± 0.45 (SD) for COVID-negative patients, P = 0.02. There were no statistically significant differences in any of the other post-procedure laboratory values or vital signs analyzed between COVID-positive and COVID-negative patients [Table 3].
The number of post-procedure deaths was 5 (35.71%) for COVID-positive patients and 6 (23.08%) for COVID-negative patients, P = 0.40 as of chart review conducted in September 2020. The number of procedural complications was 2 (14.29%) for COVID-positive patients and 4 (15.38%) for COVID-negative patients, P = 0.93. Of the two procedural complications for COVID-positive patients, 0 resulted in death. Of the four procedural complications for COVID-negative patients, two resulted in death [Table 3].
DISCUSSION
Image-guided percutaneous cholecystostomy tube placement is an established treatment for the management of acute cholecystitis and acalculous cholecystitis in patients who are poor surgical candidates or as a bridge to an eventual surgical cholecystectomy.[2-9] The accepted indications for a percutaneous cholecystostomy tube are typically right upper quadrant abdominal pain, fever, elevated WBC count, and confirmed imaging findings of cholecystitis.
During the peak pandemic months, prolonged ICU stays in COVID-positive patients resulted in an increased rate of intubation, with acalculous cholecystitis being one of the known complications which develops during lengthy ICU stays.[10] As a consequence, a significant increase in the relative number of IR consults for cholecystostomy tube placement during the peak pandemic months was observed.
Many COVID-positive patients referred for cholecystostomy tubes had a fever and elevated WBC count. Because several patients were intubated at the time of their initial imaging examination, assessment of abdominal pain or clinical sonographic evidence of Murphy’s sign was limited. When comparing pre-procedural clinical variables of COVID-positive and COVID-negative patients who received a cholecystostomy tube, our study found a statistically significant difference only when comparing the intubation status of patients during their ICU stay for COVID pneumonia. Post-procedurally, there was no statistically significant difference in mortality, procedural complications, or positive microbiology cultures following cholecystostomy tube placement between COVID-positive and COVID-negative patients.
CONCLUSION
Percutaneous cholecystostomy tube placement is likely a safe treatment for acalculous cholecystitis in patients with COVID-19. Furthermore, there were no significant differences in pre-procedural imaging findings, suggesting that COVID-19 may not directly cause cholecystitis but may be indirectly responsible for acalculous cholecystitis given the increased incidence of intubation and ICU admissions in COVID-positive patients.
The changes introduced in personnel redeployment and the management of operative candidates as a result of the COVID-19 pandemic complicates the interpretation of the surge in referrals to IR for percutaneous cholecystostomy tube placement. Furthermore, 10 (38.5%) COVID-negative patients and 8 (57.1%) COVID-positive patients who received a cholecystostomy tube were in the ICU. This could have contributed to an increased incidence of ultrasound imaging during their ICU stay, increasing the probability of finding imaging evidence of acalculous cholecystitis, leading to increased cholecystostomy tube placement, particularly in those with a fever and elevated WBC count.
Finally, although not all patients during the pandemic period met imaging criteria for acute cholecystitis, many had clinical criteria suspicious for cholecystitis, prompting cholecystostomy tube requests to eliminate cholecystitis as a source of fever. Moreover, the diagnostic criteria for acute cholecystitis in patients from 2019 were not available for comparison, which is an important limitation of the study in addition to the small sample size. Future studies will assess whether the diagnostic criteria for acute cholecystitis have changed.
Acknowledgments
We would like to thank the many collaborative discussions and aid of the MGH IR/DR research group, irlab.mgh. harvard.edu.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Som is a founder, board member, and chief medical officer of CareSignal, a digital health company. Dr. Uppot reports grants from ACR Innovation and non-financial support from Case Western Hololens Anatomy, outside the submitted work. Dr. Daye, Dr. Di Capua, Austin Snyder, Nicholas Reid, Silvia Salamone, and Tristan Yeung declare no conflicts of interest.
References
- Learning from Global Experience (IRC-101) 2020. Gest. Available from: https://www.elearn.gestweb.org/catalog/info/id:135 [Last accessed on 2020 May 06]
- [Google Scholar]
- Percutaneous cholecystostomy for acute cholecystitis in critically ill patients. Surgery. 1997;121:398-401.
- [CrossRef] [Google Scholar]
- Ultrasound-guided percutaneous cholecystostomy as an initial treatment for acute cholecystitis in elderly patients. Dig Surg. 1998;15:328-32.
- [CrossRef] [Google Scholar]
- Effective use of percutaneous cholecystostomy in high-risk surgical patients: Techniques, tube management, and results. Arch Surg. 1999;134:727-31. discussion 731-2
- [CrossRef] [Google Scholar]
- Emergency cholecystostomy and subsequent cholecystectomy for acute gallstone cholecystitis in the elderly. Br J Surg. 1999;86:1521-5.
- [CrossRef] [Google Scholar]
- Selective use of tube cholecystostomy with interval laparoscopic cholecystectomy in acute cholecystitis. Arch Surg. 2000;135:341-6.
- [CrossRef] [Google Scholar]
- Percutaneous cholecystostomy is an effective treatment for high-risk patients with acute cholecystitis. Am Surg. 2000;66:33-7.
- [Google Scholar]
- Percutaneous cholecystostomy for acute cholecystitis in high-risk patients. Ann Chir. 2000;125:738-43.
- [CrossRef] [Google Scholar]
- Gastrointestinal complications in critically Ill patients with COVID-19. Ann Surg. 2020;272:e61-2.
- [CrossRef] [Google Scholar]






