Translate this page into:
Comparison of percutaneous ethanol injection and radiofrequency ablation for the treatment of hepatocellular carcinoma

*Corresponding author: Eric Mastrolonardo, Department of Radiology, Kaiser Permanente Los Angeles Medical Center, Los Angeles, California, United States. evm004@jefferson.edu
-
Received: ,
Accepted: ,
How to cite this article: Mastrolonardo E, Jia JB, Eng B, Sakioka J, Lam C, Der D. Comparison of percutaneous ethanol injection and radiofrequency ablation for the treatment of hepatocellular carcinoma. Am J Interv Radiol 2020;4:16.
Abstract
Objectives:
The objectives of the study were to compare overall survival (OS) and disease-free survival (DFS) following percutaneous ethanol injection (PEI) and radiofrequency ablation (RFA) for the treatment of hepatocellular carcinoma (HCC).
Material and Methods:
This is a single-institution retrospective cohort study. Patients who underwent PEI or RFA between January 1, 2008, and December 31, 2009, for HCC were included in this study. Patient data were collected from the time of their procedure to October 31, 2017. One hundred and twenty-five patients received RFA or PEI during the study period. Twenty-one patients were excluded from the study because they received RFA or PEI for non-HCC cancers, leaving 47 patients in the PEI group and 57 patients in the RFA group. Primary endpoints were OS and DFS following PEI or RFA. Secondary endpoints included rates of secondary intervention and liver transplant. Statistical analysis was performed using SAS Enterprise Guide 7.13 (Cary, NC).
Results:
One-hundred and four patients are included in this study: 47 in the PEI group and 57 in the RFA group. At 9-year follow-up, the OS rates were not statistically significant between the RFA and PEI groups, 23.9% and 22.8%, respectively (P = 0.25). However, at earlier time points, there was a statistically significant difference between the two groups with higher rates of OS in the RFA group (Wilcoxon, P = 0.04). Patients in the RFA group had OS rates of 56.1%, 43.9%, and 35.1% at 3, 5, and 7 years, respectively, compared to the PEI rates of 36.4%, 27.3%, and 25.1% at those same time points (P = 0.0035). The RFA group had 29% decreased risk of death at 5 years compared to PEI based on the Cox proportional hazards model. The DFS was not significantly different between the two groups at all-time points (P = 0.96). The PEI group showed DFS rates of 32.4% at 3 years and 29.5% at 5, 7, and 9 years. The RFA group demonstrated DFS rates of 32.2% at 3 years, 26.3% at 5 years, 23.4% at 7 years, and 19.5% at 9 years.
Conclusion:
RFA and PEI have comparable 9-year OS and DFS in patients with HCC. However, at earlier time points, RFA has superior OS.
Keywords
Hepatocellular carcinoma
Percutaneous ethanol injection
Radiofrequency ablation
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common type of liver cancer and is the third leading cause of cancer mortality worldwide. Management and treatment of HCC results in an annual economic burden of greater than 400 million dollars in the United States.[1] The incidence of HCC in the United States is estimated at six cases per hundred thousand patients-year.[2] With a 5-year survival rate across all disease stages estimated between 10 and 20%, disease prognosis for HCC is poor.
The majority of patients with HCC have cirrhosis. Typically, cancer prognosis is largely determined by tumor staging, but for patients with HCC, cirrhosis is also a key risk factor.[3,4] While a number of staging systems exist, the Barcelona Clinic Liver Cancer (BCLC) system is considered the standard staging system for clinical management; it takes into account tumor burden, performance status, and severity of cirrhosis. While surgical resection is the standard treatment of early stage tumors based on the BCLC system, non-surgical interventions are recommended for high-risk surgical candidates due to poor hepatic reserves or multifocal disease.[3,5,6] Non-surgical interventions include microwave ablation, radiofrequency ablation (RFA), and percutaneous ethanol injection (PEI). Multiple studies have shown that RFA has comparable overall survival (OS) rates to surgical resection in patients with early stage disease.[7]
According to current international guidelines, RFA is the treatment of choice for local ablation.[3] However, PEI is an alternative modality. The previous studies comparing these two types of ablation have shown mixed results. The randomized control trial performed by Shiina et al. who showed that RFA was superior to PEI in terms of OS at 4 years in patients with HCC with lesions 3 cm.[8] However, multiple studies have demonstrated different outcomes in the two treatments resulting in similar OS rates.[9-11] These studies had a maximum observation period of 5 years, and OS and disease-free survival (DFS) were not always the primary endpoints. The purpose of this retrospective study is to provide our single-institution experience and describe our long-term outcomes (OS and DFS) using both RFA and PEI for more than 2 decades.
MATERIAL AND METHODS
This is a single-institution retrospective cohort study approved by the Institutional Review Board. Patients included underwent PEI or RFA between January 1, 2008, and December 31, 2009, for the treatment of HCC. Patient data were collected from the time of the procedure to October 31, 2017. Statistical analysis was performed using SAS Enterprise Guide 7.13 (Cary, NC). Patients were identified by searching the electronic medical record for current procedural terminology (CPT) codes.
One hundred and twenty-five patients received RFA or PEI during the study period. Twenty-one patients were excluded from the study because they received RFA or PEI for nonHCC cancers including cholangiocarcinoma, melanoma, and metastatic breast and colon cancers. This resulted in 47 patients in the PEI group and 57 patients in the RFA group. All qualifying patients’ charts were reviewed for baseline characteristics, outcomes data, and any inclusion or exclusion criteria beyond the scope of CPT code identifier. Primary endpoints were OS and DFS following PEI or RFA. Secondary endpoints included rates of secondary intervention and liver transplant.
At our institution, PEI is generally considered for patients who are not optimal candidates for RFA because of one or more of the following:
Markedly elevated bilirubin ≥6 mg/dL
Close proximity to surrounding organs determined by anticipated size of ablative therapy or the presence of large bile ducts or large vessels which would be at risk of thermal injury and heat sink
Tumors larger than 3 cm.
All patient treatment plans were discussed during the weekly multidisciplinary liver tumor board. PEI was administered in a single session. The maximum volume injected was based on the volume of a sphere: 4/3πr3. However, maximum volume was not always reached as we reimaged at 5 mL intervals to check the distribution of EtOH. If the entire tumor was infiltrated with EtOH, we stopped infusing regardless of volume already administered. For RFA ablative technique, we followed LeVeen’s manufacturer protocol (Boston Scientific). Probe sizes used were 2 cm, 3 cm, and 3.5 cm. Burning was continued with increasing wattage until roll-off or 30 min, whichever came first. This was typically done for 2 cycles.
Statistical analysis
For the bivariate analysis, Chi-square/Fisher’s exact tests and t-test/Wilcoxon rank-sum test were used for categorical and continuous data, respectively. Multivariate logistic regression was applied to detect the independent factors associated with the binary outcome of secondary intervention rate. Mortality following intervention was analyzed using Kaplan–Meier estimates and multivariate Cox proportional hazard model was applied for a 5- and 7-year follow-up period using the same predictor variables in the logistic regression.
RESULTS
Baseline characteristics are depicted in Table 1. The median age of the PEI and RFA groups is 63 and 61, respectively (P = 0.9). Both groups had a higher proportion of males; PEI group had 35 (74%) males and RFA group had 45 (81%) males. The difference between the two groups was not statistically significant (P = 0.4). The rates of hepatitis B and C and number of tumors were similar between the two groups. The PEI group had a significantly higher percentage of patients with alcoholic liver cirrhosis, tumors ≥3 cm, and Child–Pugh B or C liver cirrhosis. The PEI group had 33 (70%) patients with tumors ≥3 cm; the RFA group had 25 (44%) patients with tumors ≥3 cm (P = 0.007). The rate of Child–Pugh B liver cirrhosis was 40% in the PEI group (19 patients) and 25% in the RFA group (14 patients). Seven (15%) patients in the PEI group were categorized as Child–Pugh C while 0 (0%) patient in the RFA group was categorized as Child–Pugh C (P = 0.007). The median follow-up period of the PEI and RFA groups was 1.7 years and 3.6 years, respectively (P = 0.035). Notably, the RFA cohort had a significantly higher percentage of patients with a history of prior treatments for their HCC ‒ 83% (47 patients) versus 57% (27 patients) in the PEI cohort (P = 0.005). Total prior treatments included unlisted treatments (51%), transarterial chemoembolization (TACE) alone (30%), resection alone (5.7%), RFA alone (3.4%), resection and TACE (3.4%), TACE and PEI (2.3%), PEI alone (1.1%), resection and RFA (1.1%), and TACE and RFA (1.1%). There was no significant difference in subgroup analysis for these different prior treatments.
| Procedure | PEI (n=47) | RFA (n=57) | Total (n=104) | P-value |
|---|---|---|---|---|
| Baseline charac. | ||||
| Age | 0.53351 | |||
| N | 47 | 57 | 104 | |
| Mean (SD) | 61.9 (10.12) | 63.1 (10.24) | 62.5 (10.16) | |
| Range | (29.0–78.0) | (42.0–85.0) | (29.0–85.0) | |
| Sex | 0.58952 | |||
| F | 12 (25.5%) | 12 (21.1%) | 24 (23.1%) | |
| M | 35 (74.5%) | 45 (78.9%) | 80 (76.9%) | |
| Cirrhosis | 0.12453 | |||
| N | 1 (2.1%) | 6 (10.5%) | 7 (6.7%) | |
| Y | 46 (97.9%) | 51 (89.5%) | 97 (93.3%) | |
| Hep_B | 0.90942 | |||
| N | 35 (74.5%) | 43 (75.4%) | 78 (75%) | |
| Y | 12 (25.5%) | 14 (24.6%) | 26 (25%) | |
| Hep_C | 0.59512 | |||
| N | 19 (40.4%) | 26 (45.6%) | 45 (43.3%) | |
| Y | 28 (59.6%) | 31 (54.4%) | 59 (56.7%) | |
| Alcoholic* | 0.01692 | |||
| N | 30 (63.8%) | 48 (84.2%) | 78 (75%) | |
| Y | 17 (36.2%) | 9 (15.8%) | 26 (25%) | |
| Prior_tx* | 0.00512 | |||
| N | 20 (42.6%) | 10 (17.5%) | 30 (28.8%) | |
| Y | 27 (57.4%) | 47 (82.5%) | 74 (71.2%) | |
| Pugh* | 0.00043 | |||
| A | 21 (44.7%) | 43 (75.4%) | 64 (61.5%) | |
| B | 19 (40.4%) | 14 (24.6%) | 33 (31.7%) | |
| C | 7 (14.9%) | 0 (0%) | 7 (6.7%) | |
| Multi_tumor | 0.49812 | |||
| N | 35 (74.5%) | 39 (68.4%) | 74 (71.2%) | |
| Y | 12 (25.5%) | 18 (31.6%) | 30 (28.8%) | |
| Tumor_size_less3* | 0.00712 | |||
| N | 33 (70.2%) | 25 (43.9%) | 58 (55.8%) | |
| Y | 14 (29.8%) | 32 (56.1%) | 46 (44.2%) | |
| Results | ||||
| Add_proc | 0.05762 | |||
| Missing | 1 (%) | 0 (%) | 1 | |
| N | 23 (50%) | 18 (31.6%) | 41 (39.8%) | |
| Y | 23 (50%) | 39 (68.4%) | 62 (60.2%) | |
| Resection | 0.16973 | |||
| Missing | 1 (%) | 0 (%) | 1 | |
| N | 42 (91.3%) | 56 (98.2%) | 98 (95.1%) | |
| Y | 4 (8.7%) | 1 (1.8%) | 5 (4.9%) | |
| Percut_tx | 0.25572 | |||
| N | 39 (83%) | 42 (73.7%) | 81 (77.9%) | |
| Y | 8 (17%) | 15 (26.3%) | 23 (22.1%) | |
| Locoreg_tx* | 0.00332 | |||
| N | 37 (78.7%) | 29 (50.9%) | 66 (63.5%) | |
| Y | 10 (21.3%) | 28 (49.1%) | 38 (36.5%) | |
| Transplant | 0.85942 | |||
| Missing | 1 (%) | 0 (%) | 1 | |
| N | 34 (73.9%) | 43 (75.4%) | 77 (74.8%) | |
| Y | 12 (26.1%) | 14 (24.6%) | 26 (25.2%) | |
| Chemo* | 0.04482 | |||
| Missing | 1 (.%) | 0 (.%) | 1 | |
| N | 38 (82.6%) | 37 (64.9%) | 75 (72.8%) | |
| Y | 8 (17.4%) | 20 (35.1%) | 28 (27.2%) | |
| fu_year* | 0.03494 | |||
| N | 47 | 57 | 104 | |
| Mean (SD) | 3.6 (3.79) | 4.8 (3.57) | 4.3 (3.70) | |
| Median | 1.7 | 3.6 | 2.5 | |
| Q1, Q3 | 0.8, 5.5 | 1.5, 8.0 | 1.2, 7.9 | |
| Range | (0.3-12.0) | (0.2-12.5) | (0.2-12.5) |
Both groups had comparable mortality rates during the follow-up period, 76.6% in the PEI group and 78.9% in the RFA group (P = 0.25). Median survival for RFA was 3.6 years while PEI was 1.9 years. Furthermore, disease recurrence was similar between the two groups: RFA 68.4% versus PEI 63.8% (P = 0.96). About 68% of patients in the RFA group required additional treatment versus 50% of patients in the PEI group (P = 0.058). Patients in the RFA group had more subsequent locoregional treatment (49.1% vs. 21.3%, P = 0.0033) and chemotherapy (35.1% vs. 17.4%, P = 0.045) compared to the PEI group.
The Kaplan–Meier survival graphs show that OS was similar at 9 years [Figure 1]. For the RFA group, OS at 9 years was 23.9%, and OS in the PEI group was 22.8% (log-rank test, P = 0.25). However, at earlier time intervals, there was a significant difference in OS between the two groups (Wilcoxon, P = 0.04). Patients in the RFA group had OS rates of 56.1%, 43.9%, and 35.1% at 3, 5, and 7 years, respectively. This is compared to the PEI rates of 36.4%, 27.3%, and 25.1% at those same time intervals. In addition, based on the Cox proportional hazards model, the RFA group had 29% decreased risk of death at 5 years compared to the PEI group [Table 2].
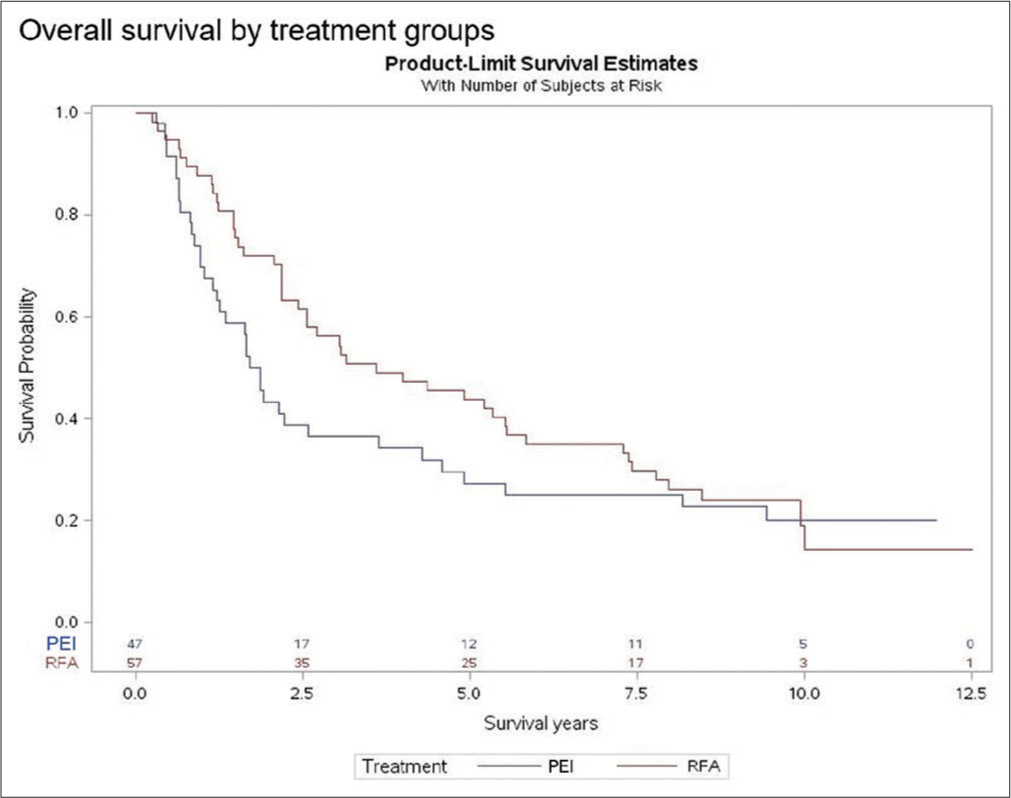
- Comparison of overall survival between RFA and PEI (Kaplan–Meier model). RFA: Radiofrequency ablation, PEI: Percutaneous ethanol injection.
| Parameter | Hazard ratio | 95% hazard ratio confidence limits | Pr>Chi-Sq | ||
|---|---|---|---|---|---|
| Treatment | RFA versus PEI | 0.709 | 0.361 | 1.396 | 0.3199 |
| AGE | 0.996 | 0.969 | 1.024 | 0.7732 | |
| Sex | M versus F | 1.199 | 0.598 | 2.402 | 0.6095 |
| Cirrhosis | Y versus N | 0.664 | 0.157 | 2.814 | 0.5785 |
| Hep_B | Y versus N | 0.752 | 0.285 | 1.982 | 0.5647 |
| Hep_C | Y versus N | 1.785 | 0.73 | 4.369 | 0.2043 |
| Alcoholic | Y versus N | 1.333 | 0.6 | 2.96 | 0.4807 |
| Prior_tx | Y versus N | 1.4 | 0.689 | 2.844 | 0.3519 |
| Child–Pugh* | A versus BC | 0.512 | 0.26 | 1.01 | 0.0534 |
| Multi_tumor | Y versus N | 1.605 | 0.857 | 3.006 | 0.1396 |
| Tumor_size_less3* | Y versus N | 0.496 | 0.278 | 0.886 | 0.0178 |
| Add_proc | Y versus N | 0.643 | 0.234 | 1.772 | 0.3935 |
| Resection | Y versus N | 1.109 | 0.262 | 4.703 | 0.8881 |
| Percut_tx | Y versus N | 0.875 | 0.41 | 1.868 | 0.731 |
| Locoreg_tx | Y versus N | 1.187 | 0.427 | 3.3 | 0.7432 |
| Transplant* | Y versus N | 0.258 | 0.101 | 0.656 | 0.0044 |
| Chemo* | Y versus N | 0.482 | 0.244 | 0.952 | 0.0357 |
There was no significant DFS difference between the two groups at all-time points based on Kaplan–Meier estimates (log-rank test, P = 0.96) [Figure 2]. Patients in the PEI group showed DFS rates of 32.4% at 3 years and 29.5% at 5, 7, and 9 years. In comparison, patients in the RFA group demonstrated DFS rates of 32.2% at 3 years, 26.3% at 5 years, 23.4% at 7 years, and 19.5% at 9 years.

- Comparison of disease-free survival between RFA and PEI (Kaplan–Meier model). RFA: Radiofrequency ablation, PEI: Percutaneous ethanol injection.
Transplantation rates during the follow-up period were comparable between the two groups ‒ 26% (PEI) and 25% (RFA). Secondary analyses were performed with stratification by tumor size, tumor number, etiology of cirrhosis (alcoholic status and hepatitis B and C), clinical scoring of cirrhosis (Child–Pugh scores), and history of prior treatment to control for confounding variables and mitigate differences in baseline characteristics. Stratified analysis showed that for patients with tumors >3 cm and Child–Pugh A liver cirrhosis, RFA had superior OS at earlier time intervals. For patients with tumors ≤3 cm and Child– Pugh B or C liver cirrhosis, there was no difference in OS at all-time intervals between the two treatment groups. In treatment-naïve patients, OS was not statistically significant between the two groups (log-rank test, P = 0.95). Patients with a history of prior treatment had significantly higher OS in the RFA group than PEI group.
DISCUSSION
Our analysis demonstrates that patients who have not had prior treatment, have tumors <3 cm, or are Child–Pugh Class B or C have comparable OS at 9 years when treated with PEI or RFA. By comparison, patients outside these specifications (i.e., patients who have had prior treatment or are Child– Pugh A) have increased OS with RFA treatment. In the overall study groups, the RFA cohort demonstrated increased OS over PEI up to 7 years. Our results suggest that both treatments are comparable for small tumors. Prior studies have had conflicting results regarding OS in patients with small tumors. For example, randomized control trials performed by Lin et al. and Shiina et al. who showed that RFA had higher OS compared to PEI for tumors ≤3 cm over 3-year and 4-year observation periods, respectively. Conversely, Pompili et al. in a retrospective analysis found no difference in 5-year OS in patients with tumors <2 cm.[12]
Our results showed similar DFS between the RFA and PEI treatment groups at all-time points (P = 0.9420). This is in contrast to the randomized control trial performed by Lin et al. which demonstrated increased DFS in patients treated with RFA at up to 3 years.[13] These differences in OS and DFS results found in our study compared to the prior literature are likely multifactorial. The aforementioned studies were performed in different patient populations and medical systems. Lin et al.’s large retrospective study was performed using Taiwan’s database and Pompili et al.’s study was performed in Italy.[12,13] Patient characteristics, physician technique, and clinical care algorithm thus may vary from our US population. In addition, Pompili et al.’s study time period began in 1988 at which time treatment techniques and available instruments and treatment algorithms likely differed.[12]
The major strengths of this study are the 4.3-year mean follow-up period and the current study population. Lencioni, Brunello, and Lin et al. had follow-up periods of 2 years or less. The major RCTs looking at PEI were published more than 10 years ago. This study is a reflection of outcomes of RFA and PEI in our current practice with a contemporary patient population and accounting for current technologies.
It is valuable to continue to reevaluate therapies as time goes by since systems, technology, and training progress and change.[8,10,13] Study limitations include single-center cohort and retrospective study design which limited standardization of groups. Unlike prior studies, our study included patients regardless of Child–Pugh score which added to the heterogeneity of the patient groups. Our intent was to present our single-center experience using these two procedures over an extended period of time as well as to give an accurate presentation of cases and their clinical outcomes. Stratified analysis was performed to mitigate baseline differences in secondary analyses.
Our data point to several criteria which could aid in stratifying patients before choosing between RFA and PEI for the treatment of HCC. Patients with Child–Pugh A may benefit from treatment with RFA. By comparison, in patients who are treatment-naïve and with small tumors or more severe stage cirrhosis, clinicians may choose between the two treatments with similar outcomes.
In the future, it will be advantageous to look at the combination of these ablative therapies with the newer systemic therapies developed for the treatment of advanced HCC for improved survival and tumor control.
CONCLUSION
Overall, there is comparable long-term OS and DFS in patients receiving RFA or PEI for the treatment of HCC.
Declaration of patient consent
Institutional Review Board permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- The burden of illness associated with hepatocellular carcinoma in the United States. J Hepatol. 2009;50:89-99.
- [CrossRef] [PubMed] [Google Scholar]
- Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol. 2014;109:542-53.
- [CrossRef] [PubMed] [Google Scholar]
- AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-80.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519-24.
- [CrossRef] [PubMed] [Google Scholar]
- Clarification of risk factors for abdominal operations in patients with hepatic cirrhosis. Ann Surg. 1984;199:648-55.
- [CrossRef] [PubMed] [Google Scholar]
- Surgery in the patient with liver disease. Trans Am Clin Climatol Assoc. 2010;121:192-204.
- [Google Scholar]
- Transarterial chemoembolization monotherapy in combination with radiofrequency ablation or percutaneous ethanol injection for hepatocellular carcinoma. Asian Pac J Cancer Prev. 2016;17:4349-52.
- [Google Scholar]
- A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122-30.
- [CrossRef] [PubMed] [Google Scholar]
- Small hepatocellular carcinoma in cirrhosis: Randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235-40.
- [CrossRef] [PubMed] [Google Scholar]
- Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: A randomized controlled trial. Scand J Gastroenterol. 2008;43:727-35.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous radiofrequency ablation of hepatocellular carcinoma compared to percutaneous ethanol injection in treatment of cirrhotic patients: An Italian randomized controlled trial. Anticancer Res. 2011;31:2291-5.
- [Google Scholar]
- Single hepatocellular carcinoma smaller than 2 cm: Are ethanol injection and radiofrequency ablation equally effective? Anticancer Res. 2015;35:325-32.
- [Google Scholar]
- Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology. 2004;127:1714-23.
- [CrossRef] [PubMed] [Google Scholar]






