Translate this page into:
Abdominal Wall Fat Necrosis after Extraperitoneal Bladder Perforation

*Corresponding author: Jerel Chacko, Department of Emergency Medicine, Staten Island University Hospital, 475 Seaview Avenue, Staten Island, New York 10305, USA. jchacko3@northwell.edu
-
Received: ,
Accepted: ,
How to cite this article: Chacko J, Kondrat J. Abdominal Wall Fat Necrosis after Extraperitoneal Bladder Perforation. Am J Interv Radiol 2019;3:(11).
Abstract
Suprapubic catheters (SPCs) are a therapeutic intervention used to manage long-term urinary tract dysfunction; it is commonly used in patients who may be poor candidates for surgical intervention. Generally considered a relatively safe procedure, this case report describes a rare but serious complication of insertion of an SPC.
Keywords
Complication
Fat necrosis
Suprapubic catheter
INTRODUCTION
Suprapubic catheters (SPCs) are a therapeutic intervention used to manage long-term urinary tract dysfunction; it is commonly used in patients who may be poor candidates for surgical intervention. SPCs prevent urethral stricture and fibrosis associated with intermittent or indwelling Foley catheterization. Initial placement is an invasive procedure usually conducted by urology or interventional radiology (IR) with subsequent exchanges being performed in the outpatient setting by the provider who inserted the catheter, a trained nurse practitioner, or a visiting nurse service.[1]
Severe complications arising from SPC use commonly involve an improper exchange of the catheter in the community setting with cases of intraperitoneal, bowel, and vaginal perforation being reported.[1-4] Despite the multiple comorbidities of the typical SPC patient, insertion of an SPC is generally considered a safe procedure with a short-term complication rate of 10–29% and a 30-day mortality rate of 0.8–1.8%.[2,3] The most commonly reported complications from SPC insertion and maintenance include site bleeding, catheter blockage, malposition of the catheter, and bowel injury.[2,3,5-8] Of these, bowel injury is considered the most severe, with an incidence rate of 2.4–2.7%.[2,3] Here, we describe a case of abdominal skin and fat necrosis with associated septicemia after a failed attempt at initial insertion of an SPC.
CASE REPORT
A 71-year-old male with a history of urinary retention secondary to benign prostatic hyperplasia requiring a chronic Foley catheter, Enterococcus faecium urinary tract infection 4 months prior, seizures, chronic obstructive pulmonary disease on 3 L of home oxygen, hypertension, dyslipidemia, and peripheral vascular disease with a subsequent left below the knee leg amputation presented to the emergency department (ED) complaining of abdominal and penile pain. One hour before arrival, the patient underwent a failed attempt to insert a SPC by IR. After the attempt in IR, the SPC was removed, pressure was applied, the surgical wound was dressed, a new Foley catheter was inserted, and the patient was discharged.
In IR, a preprocedural ultrasound was done to assess the anatomical location of the bladder. The patients preexisting Foley was infused with saline where moderate distention was noted on ultrasound. Gentle negative traction was placed on the preexisting Foley to prevent leakage around the Foley and to increase distention. The patient was prepped, draped and 1% lidocaine solution was used as a local anesthetic.
A 5-French/7 cm needle/trocar sheath assembly was introduced into the bladder, the needle was removed and placement of trocar sheath confirmed through fluoroscopy with a small amount of Conray contrast. Of note, the patient had a history of anaphylactic allergic reaction to gadolinium containing compounds, an unknown allergic reaction to iodine-based contrast media, and oxycodone. A 0.0035 guidewire was advanced and confirmed to the borders of the bladder (Figure 1). A 10-French dilator was advanced over the wire and then exchanged for 10 mm × 15 cm balloon catheter to dilate the tract. As the balloon was deflated and the catheter removed, a 30-French trocar sheath was advanced over guidewire into the bladder and placement confirmed through outflow of urine/saline/ contrast. A 26-French catheter was advanced through the trocar sheath but placement appeared to be extravesicular on fluoroscopy. This was confirmed with ultrasound where the catheter was visualized in the anterior subcutaneous tissues; the catheter was removed and no drainable fluid was detected by ultrasound. The existing Foley catheter was then exchanged; since the patient refused to leave a 14-French catheter in place as a drain, the trocar sheath, and wire were removed, pressure was applied, the wound was dressed, and the patient was discharged with instructions to come to the ED if the patient experienced worsening abdominal pain, fever, or nausea.
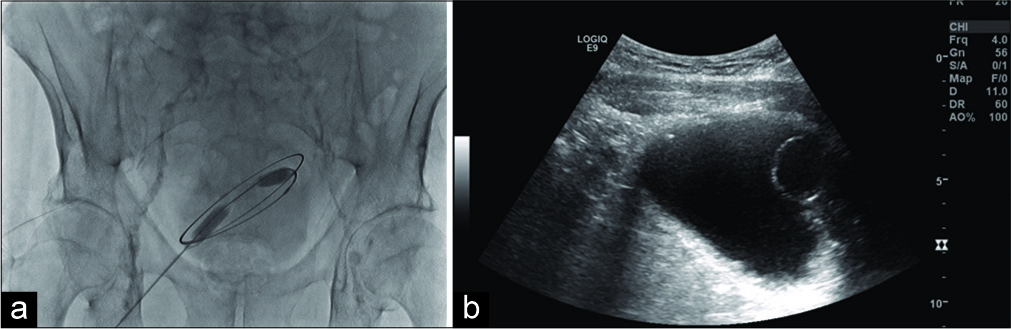
- A 71-year-old male presenting for suprapubic catheter placement. (a) Ultrasound image demonstrates Foley balloon in distended bladder. (b) Fluoroscopy image of 0.035 guidewire conforming to the borders of the bladder.
One hour later, the patient presented to the ED in severe distress due to diffuse abdominal pain which distracted him from answering questions. He was afebrile with a temperature of 35.7°C, a blood pressure of 172/80 mmHg, heart rate of 66 beats/min, respiratory rate of 26 breaths/ min, and oxygen saturation of 96% on room air. On physical examination, his abdomen was mildly distended with diffuse tenderness to palpation and peritoneal signs, normal bowel sounds, and mild erythema around the SPC puncture site. His external genitalia were normal appearing and without skin discoloration, edema, or crepitus. No hernias were present, no abnormalities were noted in the urethra, the epididymis appeared normal bilaterally, cremasteric reflex was intact, and no urethral discharge was identified.
The Foley catheter was draining yellow, non-bloody, and cloudy urine. Laboratory testing including a complete blood count, comprehensive metabolic panel, lipase, magnesium, phosphorous, coagulation studies, troponin, and venous blood gas analysis was significant for a white blood cell count of 2710 cells/µL, a lactate of 2.1 mmol/L, magnesium level of 1.5 mg/dL, troponin of 0.06 ng/mL, and a venous pH of 7.28. Blood cultures were negative for bacteria, but a urine culture was positive for Escherichia coli. Computed tomography of the patient’s abdomen and pelvis revealed the Foley balloon inflated within a decompressed bladder and a small volume of contrast within the urinary bladder (Figure 2). Soft tissue edema and foci of extraluminal air were noted in the lower abdominal and pelvic wall. In addition, a small amount of blood was noted in the right retroperitoneal space, suggesting post-operative changes.
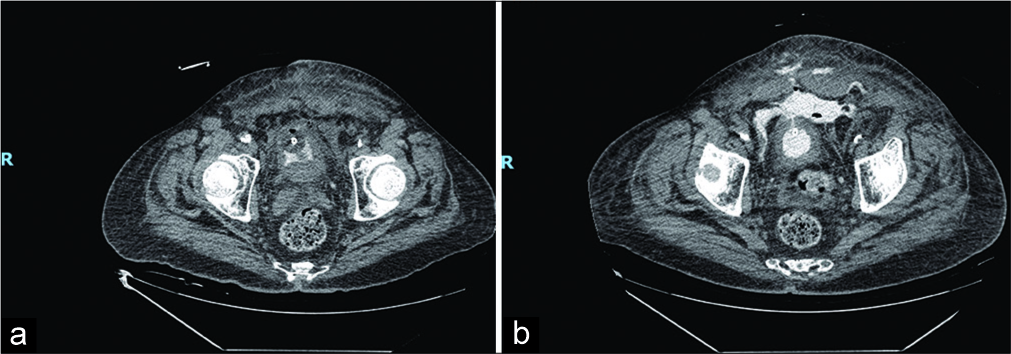
- A 71-year-old male presenting after failed suprapubic catheter placement with abdominal pain. (a) Non-contrast- enhanced computed tomography (CT) showing foci of air, consistent with recent instrumentation, and soft tissue edema of abdominal wall. (b) Repeat 12-h non-contrast-enhanced CT showing extraperitoneal contrast extravasation from the bladder extending to the subcutaneous tissues of the anterior abdominal wall through a defect seen within the urinary bladder wall.
Despite the administration of 2000 mL intravenous crystalloid, cefepime, and metronidazole, the patient’s hemodynamic state deteriorated to a blood pressure of 76/49 mmHg and a heart rate of 154 beats/min. An electrocardiogram showed sinus tachycardia. A central venous catheter was placed and norepinephrine infusion was initiated, with good response. The patient was admitted to the intensive care unit with a diagnosis of septic shock and bladder injury. Urology and infectious disease were consulted and the antibiotic regimen was changed to a 12- day course of fluconazole, meropenem, and vancomycin. Shortly after admission to the intensive care unit, the patient developed hypercapnic respiratory failure. An endotracheal tube was placed and mechanical ventilation was initiated. A repeat computed tomography of the abdomen and pelvis 12 h after admission showed extraperitoneal contrast extravasation extending to the subcutaneous tissues of the anterior abdominal wall through the defect seen within the urinary bladder. Two days after antibiotic initiation, the fluconazole was discontinued and a 10-day course of Mycamine initiated. The patient was taken for repeated skin debridement due to extensive skin sloughing, revealing soft tissue necrosis without purulence of the abdominal wall. Debridement and irrigation of multiple wounds on the trunk as well as sharp excision of the skin subcutis, pubis, and groin were performed. After the initiation, antibiotic regimen was completed, a 10-day course of piperacillin- tazobactam was administered. After the cessation of the piperacillin-tazobactam, a 7-day course of ciprofloxacin and a 21-day course of cefepime and metronidazole were initiated. The patient’s hospital stay was further complicated by a Pseudomonas aeruginosa right lower lobe ventilator- associated pneumonia. He was unable to be extubated and a tracheostomy tube and percutaneous endoscopic gastronomy tube were placed. He was started on a 21-day course of ampicillin-sulbactam; 5 days into this regimen, a 5-day course of meropenem and vancomycin was added on. After resolution of the pneumonia, he was able to be taken off mechanical ventilation. This was then followed by the development of endocarditis, for which a 6-week course of cefazolin was initiated. The patient was then discharged to a long-term rehabilitation facility. The patient is still alive and doing well.
DISCUSSION
Initial insertion of an SPC is generally considered a safe procedure with a mortality rate of 1.8%.[2] Serious complications are typically reported after exchange of the catheter during routine maintenance rather than initial insertion.[2-8] Common complications reported during initial insertion include anesthesia-related complications, inability to put patient in the lithotomy position, catheter malposition or expulsion, and bowel injury/perforation. Intraoperative complications were increased in patients with prior abdominal surgery compared to those without (31% vs. 5%).[2] One study suggested an intraoperative peritoneal injury or bowel perforation rate of 2.4%; analysis of these cases revealed improper inflation of the bladder before insertion due to small contracted bladder, patulous urethra, or prior surgical scarring of the abdominal wall as the most probable cause of improper placement.[2]
Presenting symptoms of intraperitoneal injury and bowel perforation associated with SPC placement vary and can be difficult to diagnose. The time until presentation varied from a few hours up to several weeks from initial insertion or catheter exchange.[4-9] Two case reports of peritoneal perforation showed chronic fistula formation through the bowel wall that only became symptomatic after further injury during catheter exchange and development of drainage of feculent material from the SPC.[8,10] A common symptom present was drainage of feculent or bilious material from the SPC; however, even when feculent or bilious drainage was present, some patients were asymptomatic.[8,11]
Peritoneal signs were not evident in many cases as some perforations were either confined to the peritoneum only or catheter perforation of the bowel did not lead to leakage of bowel contents into the peritoneum.[4-9] Diagnosis of SPC- related perforation is difficult as patients with SPC typically have many comorbidities that can mask the early signs of peritonitis.[12] In addition, diagnosis can be obscured as many patients and medical staff attribute abdominal and pelvic pain as the result of surgical pain from initial insertion, ileus, or soft tissue infection. In patients with an SPC exhibiting signs of peritonitis, difficulty draining the catheter, or non- clear drainage from the catheter, perforation should be considered.
In almost all cases of SPC perforation, diagnosis was made with computed tomography of the abdomen and pelvis, either with or without oral and IV contrast.[5-10,12] Other imaging modalities used plain X-ray and a fistulagram.[11]
Management of SPC perforation depended on the degree of perforation. In two cases, where the perforation was confined only to the peritoneum and not involving the bowel wall, the SPC was removed and the perforation was allowed to heal with supportive management.[4,6] Most cases of SPC perforation involving the bowel were managed with laparotomy and resection of the involved bowel.[5-8,10-12] Despite the extensive comorbidities reported in these patients, only two reported cases resulted in death of the patient.[6,11]
This patient’s pathology is likely a unique instance where bladder perforation after initial insertion of an SPC was confined to only the extraperitoneal space of the lower abdominal wall and retroperitoneum. A previously undetected E. coli colonization along with his sensitivities to contrast material may explain the rapid and severe response of septic shock. The presence of skin and fat necrosis without purulence suggests the meglumine iothalamate contrast used, instead of the infection, may be responsible for the extensive skin and skin structure damage given the patient’s known sensitivities and allergies.
Given the patients known allergy to gadolinium and iodinated contrast pre-operative diphenhydramine and a single dose of corticosteroids may have reduced the adverse abdominal wall fat reaction. Although the complications from the procedure are less likely due to an infectious etiology, urinalysis as well as prophylactic antibiotics may have been considered given his previous history of E. faecium and higher risk for UTI. Although prophylactic antibiotics are not the standard in this procedure, one study did show a reduction in post-operative infections with prophylactic antibiotic use following SPC insertion.[13]
CONCLUSION
While SPC insertion is still considered a safe procedure, this case illustrates a rare but morbid complication. Consideration as to the use of saline and ultrasound instead of contrast and fluoroscopy may be useful in the care of patients with known contrast sensitivities.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Suprapubic catheter change, what could go wrong? BMJ Case Rep. 2019;12:e229855.
- [CrossRef] [PubMed] [Google Scholar]
- The surgical risk of suprapubic catheter insertion and long-term sequelae. Ann R Coll Surg Engl. 2006;88:210-3.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term suprapubic catheterisation: Clinical outcome and satisfaction survey. Spinal Cord. 1998;36:171-6.
- [CrossRef] [PubMed] [Google Scholar]
- Bowel perforation with suprapubic cystostomy report of two cases. Obstet Gynecol. 1976;48:67S-9.
- [Google Scholar]
- Bowel Injury After a Routine Change of Suprapubic Catheter ProQuest. Available from: http://www.search.proquest.com/docview/1783274072?pq-origsite=360link [Last accessed on 2019 Jul 13]
- [Google Scholar]
- Suprapubic catheter displacement: A forgotten phenomenon. Emerg Med Aust. 2010;22:249-51.
- [CrossRef] [PubMed] [Google Scholar]
- Iatrogenic direct rectal injury: An unusual complication during suprapubic cystostomy (SPC) insertion and its laparoscopic management. Arch Ital Urol Androl. 2013;85:101-3.
- [CrossRef] [PubMed] [Google Scholar]
- Small bowel injury after suprapubic catheter insertion presenting 3 years after initial insertion. BMJ Case Rep. 2013;2013:bcr2013201436.
- [CrossRef] [PubMed] [Google Scholar]
- Occult transfixation of the sigmoid colon by suprapubic catheter. Age Ageing. 2002;31:407-8.
- [CrossRef] [PubMed] [Google Scholar]
- Delayed bowel perforation following suprapubic catheter insertion. BMC Urol. 2004;4:16.
- [CrossRef] [PubMed] [Google Scholar]
- A rare and serious complication of elective suprapubic catheter change. Uro Today Int J. 2009;2:1-3.
- [CrossRef] [Google Scholar]
- British association of urological surgeons' suprapubic catheter practice guidelines. BJU Int. 2011;107:77-85.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized, double-blind, placebo-controlled comparison of the effect of nitrofurantoin monohydrate macrocrystals on the development of urinary tract infections after surgery for pelvic organ prolapse and/or stress urinary incontinence with suprapubic catheterization. Am J Obstet Gynecol. 2004;191:182-7.
- [CrossRef] [PubMed] [Google Scholar]







