Translate this page into:
Abernethy Malformation – a Rare but Important Diagnosis Prior to Liver-Directed Therapy for Hepatocellular Carcinoma

*Corresponding author: Nainesh Parikh, 12902 USF Magnolia Drive, WCB-RAD MD/OPI, Tampa, FL 33612, USA. nainesh.parikh@moffitt.org
-
Received: ,
Accepted: ,
How to cite this article: Parikh N, Jiang K, Truong K. Abernethy Malformation – a Rare but Important Diagnosis Prior to Liver-Directed Therapy for Hepatocellular Carcinoma. Am J Interv Radiol 2019;3:9.
Abstract
A 45-year-old man with incidentally discovered, unresectable HCC were treated with TACE to the left hepatic lobe and TARE to the right hepatic lobe. Upon retrospective review, he was found to have a congenital extrahepatic portosystemic shunt with the absence of the portal vein (Abernethy malformation). This case report discusses variant splanchnic and portal anatomy in the setting of rare, congenital portosystemic shunts and evaluates types of liver-directed therapies for HCC in this setting.
Keywords
Abernathy
Portosystemic shunt
HCC
INTRODUCTION
Abernethy malformation represents a rare vascular anomaly consisting of a congenital extrahepatic portosystemic shunt (CEPS) that diverts portal venous blood from the liver into the systemic circulation and is classified by drainage pattern.[1] These shunts typically empty into the systemic circulation via the inferior vena cava (IVC), however, connections to the renal, iliac and azygous veins or the right atrium have been described.[2,3] Patients with CEPS can present in several different ways. Liver function may often be well preserved and patients typically do not develop cirrhosis. The clinical manifestations can be broadly divided into features related to portal venous blood shunting with associated symptoms, associated congenital anomalies such as cardiac, biliary and splenic malformations, or secondary to hepatocellular lesions such as hepatocellular carcinoma (HCC).[4] The etiology for the increased incidence of hepatic lesions in patients with CEPS is unclear but several theories have been proposed including hepatic ischemia with a compensatory increase in arterial flow, decreased growth factors and hormones and B-catenin mutations.[5,6]
The management of HCC in patients who have CEPS should be via standard pathways or guidelines (e.g. Barcelona Clinic Liver Cancer criteria (BCLC) or National Comprehensive Cancer Network (NCCN)), with loco-regional therapy (transarterial chemoembolization (TACE), bland transarterial embolization (TAE) or Yttrium-90 (Y-90) radioembolization (RE)) being reserved for unresectable HCC. In this case report, a patient with multiple liver lesions representing multiple HCCs without underlying cirrhosis and incidentally found CEPS is presented. Consideration is given to the fact that CEPS was not diagnosed on cross-sectional or initial angiographic imaging, and that had it been identified, therapeutic options may have been different.
CASE REPORT
A 45-year-old man with no past medical history was referred for evaluation and treatment of biopsy-proven unresectable HCC. He was initially admitted to an outside facility with abdominal pain and was found to have multiple liver masses. Percutaneous liver biopsy of the mass demonstrated moderately differentiated HCC (G2, suspicious for lymphovascular space invasion). Percutaneous liver biopsy of the adjacent normal liver demonstrated normal non-neoplastic liver [Figure 1]. His past medical history included remote gastric surgery for ulcers. Physical exam was unremarkable other than epigastric tenderness to palpation. Liver function tests were slightly altered: Child- Pugh A (serum bilirubin 1.30 mg/dl, albumin 4.2 gm/dl, alanine transferase 58 U/L, aspartate transferase 48 U/L, alkaline phosphatase 229 U/L). Viral hepatitis screening was negative and the patient had no significant history of alcohol exposure. Alpha fetoprotein (AFP) was within normal limits at 2.8 ng/ml. The patient did not have elevated ammonia levels or clinical signs of encephalopathy.
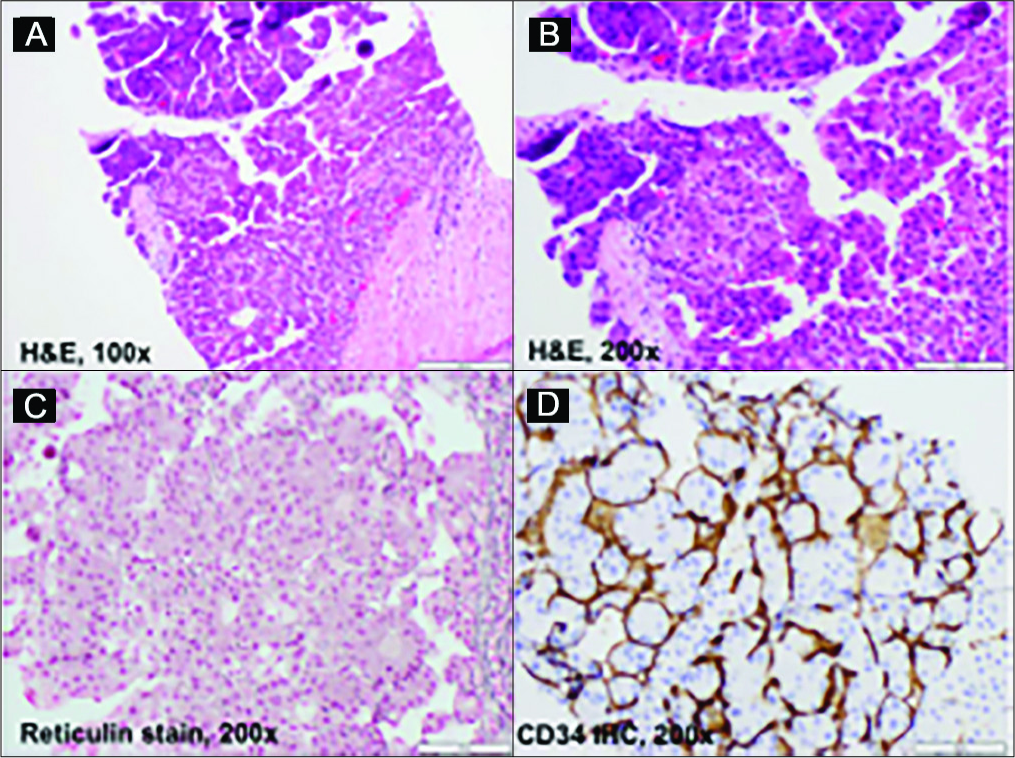
- 45-year-old man with well-differentiated hepatocellular carcinoma. Microscopic examination reveals liver tissue replaced by neoplastic enlarged hepatocytes demonstrating high nuclear to cytoplasm ratio, hyperchromatic nuclei with prominent nucleoli and irregular nuclear membrane, accompanied by occasional mitoses (A and B). These dysplastic cells were arranged in thickened cell plates, illustrated by a reticulin special stain, which is highly consistent with a neoplastic process (C). Immunostains (IHCs) performed on this biopsy demonstrate that these neoplastic cells are positive for hepatocytic antigen Heppar-1, CEA with clearly canalicular pattern, and weakly positive for alpha-fetoprotein (not shown here); CD34 IHC reveals marked endothelial wrapping of thickened cell plates (D). These findings support the diagnosis of well-differentiated hepatocellular carcinoma.
Multiphasic Eovist abdominal MRI demonstrated liver lesions with atypical enhancement characteristics of HCC within both lobes, which is common for HCC in the setting of CEPS. The largest tumor within segment 2 measured 8.6 cm and contained central necrosis with encroachment on the lesser curvature of the proximal stomach. No imaging signs of cirrhosis or portal hypertension were seen [Figure 2]. Within the report, no note was made of altered portal or systemic venous anatomy.
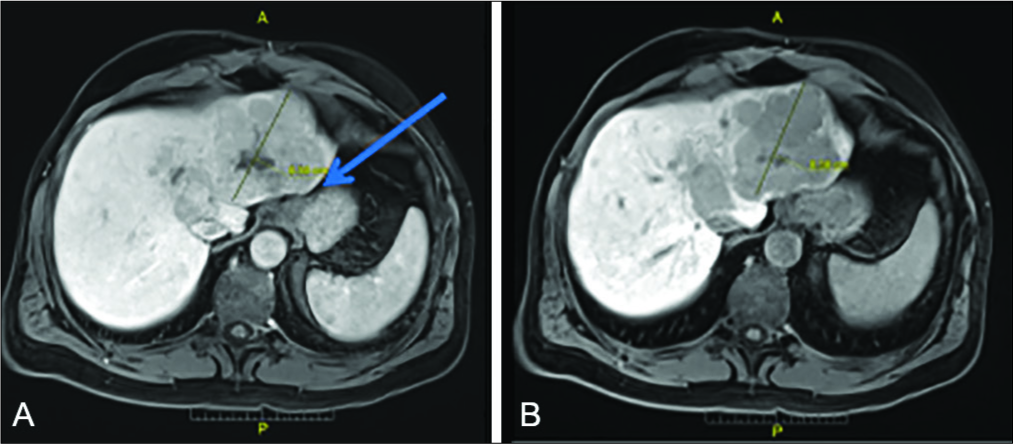
- 45-year-old man with well-differentiated hepatocellular carcinoma. Axial multiphasic MRI of the abdomen shows atypical imaging characteristics of HCC with minimal arterial enhancement (A) and lack of uptake on hepatobiliary phase imaging (B). No cirrhosis is identified and encroachment of the mass onto the lesser curvature of the stomach is seen (arrow). Notably, retrospective review of the images demonstrates no intrahepatic portal venous vasculature within either lobe.
Given the poor prognosis of the patient, he was not considered for liver transplantation outside of Milan criteria. He was discussed at a multidisciplinary tumor board, from which he was referred for liver-directed loco- regional treatment with transarterial radioembolization (TARE) for his BCLC intermediate stage (B) disease. Upon angiographic evaluation during his TARE work-up, he was found to have hypertrophied hepatic arteries with the right hepatic artery directly replaced from the aorta. Delayed images of the mesenteric venous return demonstrated patent SMV and splenic vein, without clear visualization of the intrahepatic portal veins [Figure 3]. This was thought to be due to extensive tumor burden, and a portal venous vascular malformation was not considered. Lung shunt fraction after Tc99m-MAA SPECT was 6.1%.
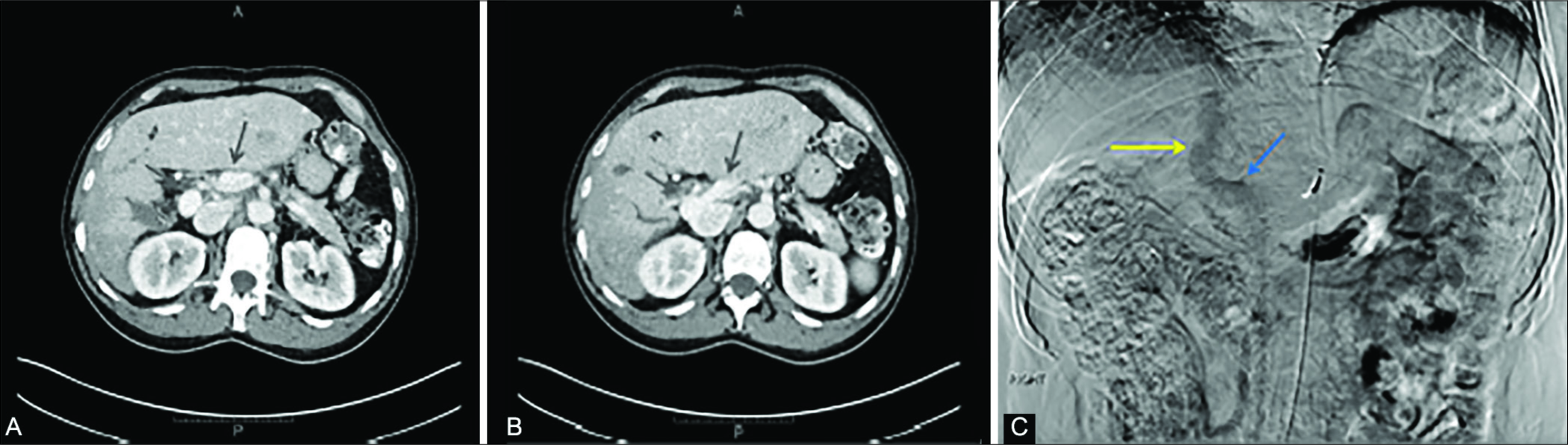
- 45-year-old man with well-differentiated hepatocellular carcinoma and congenital extra-hepatic portosystemic shunt (CEPS). Axial contrast enhanced CT images during portal venous phase demonstrate the relationship of the SMV (blue arrow), splenic vein (green arrow) and IVC (yellow arrow) inferior to the expected location of the portal confluence (A). More superiorly (B), the SMV (blue arrow) and splenic vein (green arrow) can be seen emptying directly into the IVC which has a large caliber (B), compatible with type IB CEPS. (C) Mesenteric phase after digital subtraction angiography via the superior mesenteric artery demonstrates direct drainage of the SMV (blue arrow) into the IVC (yellow arrow).
At this point, the case was discussed at weekly dedicated TARE multi-disciplinary tumor board, and the radiation oncologist calculating the dose raised a concern about the proximity of the left lobe tumor to the gastric body. While never reported within the literature, anecdotal reports of post-TARE gastro-hepatic fistula were discussed thus raising enough concern for the radiation oncologist to recommend TACE to the left lobe and TARE to the right lobe. The CEPS malformation was still undiagnosed at this point and therefore the patient was scheduled for TACE to the left lobe.
The left hepatic artery was selected with a 2.8 Fr microcatheter (Cantata, Cook, Bloomingdale, USA) and 150 mg Doxorubicin loaded onto 70–150 μ beads (LC Beads, BTG, London, United Kingdom) was delivered. Immediately after delivery while the patient was still on the angiography table, the patient developed severe nausea and vomiting with pain. The patient was admitted for supportive care, and experienced severe nausea and vomiting for 48 hours with resolution of symptoms and discharge on postoperative day 6. Liver enzymes were abnormal and consistent with acute liver injury while the patient was in the hospital, with total bilirubin rising to a peak of 2.3 mg/dl, alanine transferase rising to a peak of 2641 U/L, and aspartate transferase rising to a peak of 3149 U/L. All lab values were back to baseline before discharge.
The patient returned 6 weeks later for right lobe TARE and 1.51 GBq Y90-labeled glass-based microspheres (TheraSpheres, BTG, London, United Kingdom) were delivered. At 6-week follow up, the patient was found to have new extra-hepatic disease with a 4.2 cm right acetabular osseous metastasis and no significant in appearance of the liver tumors. The patient received radiation therapy to the right acetabulum and on single-agent Sorafenib. He then transferred his care to an outside facility and died 6 months later – 11 months after initiating liver-directed therapy. Upon retrospective review of the cross-sectional and angiographic imaging, it was clear that a direct portosystemic shunt could be visualized with direct drainage of the splenic and superior mesenteric veins into the IVC [Figure 3].
DISCUSSION
CEPS is a rare condition that is classified according to an altered splanchnic, splenic and portal venous system. In Type I, portal venous blood bypasses the liver and drains directly into the systemic circulation. This is further classified by whether or not the splenic vein and SMV are co-joined prior to drainage into the systemic circulation (Type IA) or if they drain directly into the systemic circulation (Type IB). The patient presented here demonstrated Type IB drainage with the splenic vein and SMV directly draining into the IVC. Type II CEPS is represented by hypoplastic portal venous anatomy. In adult patients with CEPS, liver function is often well preserved despite partial or complete bypass of the intrahepatic portal venous system. Elevated ammonia levels have been reported in approximately 30% of cases and hepatic encephalopathy in 10–13%.[7] Type I patients have a significantly higher prevalence of liver tumors[7] and are typically older.
The propensity for patients with CEPS to develop hepatocellular lesions is uncertain, particularly in the adult population however, hepatoblastoma and HCC have been associated with CEPS Type I. While theories exist identifying relative hepatic ischemia from disrupted portal flow as a possible etiology for increased hepatocellular lesions, this has yet to be clearly proven, even in animal models.[5,6] Other potential contributing factors for hepatocellular lesions in patients with CEPS include altered delivery of growth factors and hormones to the liver, increased arterial blood flow,[8] as well as genetic causes. For example, Sorkin et al. described the role of B-catenin mutations as a risk factor for the malignant transformation of hepatic adenomas into HCC.[5] The authors are aware of only 16 prior cases of CEPS with HCC published within the literature.[9]
Therapeutic considerations for patients with CEPS and HCCs rely on the fact that there is altered or no portal venous flow to the liver. First and foremost, pathways such as the Barcelona Clinic Liver Cancer system should be utilized for the management of known HCCs, regardless of the presence of CEPS. In addition, whether it is prior to surgical or angiographic therapy, knowledge and identification of CEPS are beneficial for planning purposes, as CEPS can be easily overlooked. In this case, attention on cross-sectional or angiographic imaging to the presence of CEPS may have allowed us to treat the patient with Y-90 to the left lobe rather than TACE, possibly allowing the patient a better therapeutic and clinical response to liver-directed therapy. As noted previously by Kroenke et al., TARE has the advantage in these patients that it can be safely performed in scenarios with absent portal venous flow such as portal vein thrombosis.[9] Likely, as those authors noted, it is related to the minimal embolic effect that does not occlude arterial supply, and instead lodges in the microvasculature of the tumor. This case report adds to the literature of TARE for HCC in a patient with CEPS, of which the authors are aware of only the one prior case reported by Kroenke et al.
Although it is difficult to draw a direct relationship between adult patients who have CEPS and an increased risk of hepatocellular lesions, it is worth noting that in young adult patients who have no risk factors for HCC – particularly those who do not have cirrhosis or hepatitis – evaluation for CEPS may be helpful. Additionally, knowledge of CEPS will help guide therapy, specifically for liver-directed therapy, with TARE serving as a safe and valuable option for first-line therapy.
CONCLUSION
In young adult patients who are diagnosed with HCC without underlying risk factors, CEPS may be a consideration of the differential diagnosis. Knowledge and awareness of this entity may lead to further diagnosis and research about its prevalence and etiology. In addition, thorough understanding of the anatomic variations in the portal vasculature in patients who have CEPS may help direct therapeutic surgical and angiographic interventions.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Congenital extrahepatic portosystemic shunts. Pediatr Radiol. 2003;33:614-20.
- [CrossRef] [PubMed] [Google Scholar]
- Congenital extrahepatic portosystemic shunt complicated by the development of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2015;14(5):552-7.
- [CrossRef] [Google Scholar]
- Type I congenital extrahepatic portosystemic shunt. J Ultrasound Med. 2009;28(5):703-5.
- [CrossRef] [PubMed] [Google Scholar]
- Congenital extrahepatic portosystemic shunt (Abernethy malformation type Ib) with associated hepatocellular carcinoma: case report and literature review. Pediatr Dev Pathol. 2017;20(4):354-62.
- [CrossRef] [PubMed] [Google Scholar]
- Multiple B-catenin mutations in hepatocellular lesions arising in Abernethy malformation. Hum Pathol. 2016;53:153-8.
- [CrossRef] [PubMed] [Google Scholar]
- Congenital extrahepatic portosystemic shunts (Abernethy malformation): a histopathologic evaluation. Am J Surg Pathol. 2011;35(9):1381-90.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical classification of congenital extrahepatic portosystemic shunts. Hepatol Res. 2010;40(6):585-93.
- [CrossRef] [PubMed] [Google Scholar]
- Insight into congenital absence of the portal vein: is it rare? World J Gastroenterol. 2008;14(39):5969-79.
- [CrossRef] [PubMed] [Google Scholar]
- Radioembolization for Hepatocellular Carcinoma Arising in the Setting of a Congenital Extrahepatic Portosystemic Shunt (Abernethy Malformation) Cardiovasc Intervent Radiol. 2018;41(8):1285-90.
- [CrossRef] [PubMed] [Google Scholar]






