Translate this page into:
Safety and Efficacy of Angio-Seal Closure in Antegrade Superficial Femoral Artery Access

Corresponding Author: William Akard, Maine Medical Center, Department of Radiology, 22 Bramhall St, Portland, ME 04101, United States. E-mail: will.akard@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Akard W, Cicuto K, Kim P, Mittleider D. Safety and Efficacy of Angio-Seal Closure in Antegrade Superficial Femoral Artery Access. Am J Interv Radiol 2018, 2(3) 1-7.
Abstract
Objective:
Arterial access for endovascular revascularization in patients with debilitating peripheral arterial disease is commonly achieved through retrograde common femoral artery (CFA) approach. However, retrograde access presents multiple technical challenges, including long distance from the access site to the target lesion, and mechanical disadvantage of working over the aortic bifurcation and often tortuous iliac vessels. Antegrade CFA access avoids these challenges but has been fraught with its own difficulties, particularly in obese patients. Antegrade superficial femoral artery (SFA) access provides the same mechanical advantages while avoiding the difficulties of antegrade CFA access, but a vascular closure device is required due to distance from the femoral head. This single-center study evaluates the safety and efficacy of the Angio-Seal device (St. Jude Medical, St. Paul, MN) in SFA punctures.
Materials and Methods:
From May 2011 to January 2015, 140 antegrade SFA punctures were performed on 110 limbs in 88 patients for endovascular revascularization, all with ultrasound guidance. Complications and patient data including age, sex, body mass index, Fontaine stage, sheath size, and intraoperative heparin doses were analyzed.
Results:
In 140 antegrade SFA punctures, there were 11 access-related complications (7.9%). The majority were hematomas or pseudoaneurysms requiring nominal or no therapy. There were 3 major complications: Two delayed access stenoses ultimately resulted in toe amputations and one hemorrhage required extended hospitalization and transfusion. Patient data analysis showed a statistically significantly increased complication rate in females (20.7%) versus males (4.5%) (p = 0.0105).
Conclusions:
Antegrade SFA access with Angio-Seal closure is safe and effective. An increased complication rate in females warrants cautious post-procedural follow-up.
Keywords
Angio-Seal
Antegrade arterial access
Endovascular revascularization
Superficial femoral artery access
Vascular closure device
INTRODUCTION
Percutaneous lower extremity arterial access for the treatment of peripheral arterial disease (PAD) is achieved commonly through a retrograde approach from the contralateral common femoral artery (CFA). Contralateral CFA access for the treatment of distal lower extremity arterial disease presents multiple challenges related to the considerable distance between the access site and the target lesion and the mechanical disadvantage of working over the aortic bifurcation, particularly in patients with tortuous iliac vessels (Figure 1a). Antegrade access has been described to address these challenges, and it has been performed traditionally using the ipsilateral CFA.[1] This technique has its own limitations: Difficulty advancing a wire into the superficial femoral artery (SFA), accidental puncture of the deep femoral artery, thickness of soft tissues from the skin to the vessel in obese patients if approaching from an oblique angle (Figure 1b and c), and kinking of the sheath at the vascular entry site if access is near perpendicular in obese patients.[2] This approach can be time consuming and lead to repeat punctures and complications such as hematomas and pseudoaneurysms.[3] The SFA may be a preferable access site in these patients. Due to its anatomic location distant from the femoral head, antegrade SFA access may not allow for safe manual compression post-procedure, and a closure device may be necessary. Relatively few studies exist examining the safety of closure devices in the SFA.[4-6] The goal of this study was to evaluate the safety and efficacy of a closure device in antegrade SFA access in an outpatient population treated for PAD.
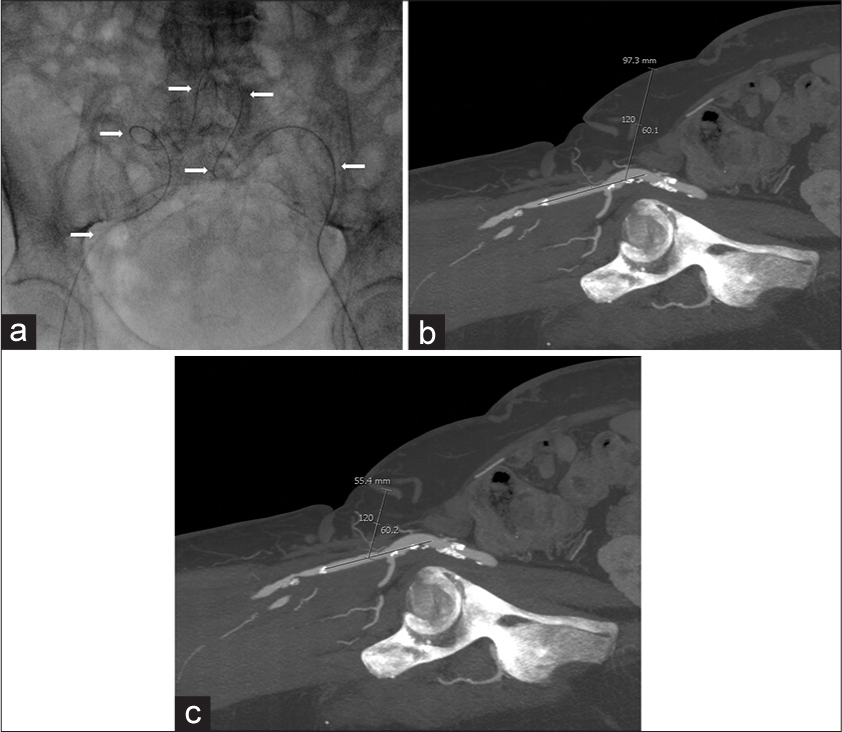
- 60-year-old woman with atherosclerosis who presented with rest pain in her legs bilaterally, with anatomy demonstrating the typical challenges of common femoral artery (CFA) access. (a) Spot anteroposterior fluoroscopic image of the pelvis shows a guidewire (arrows) extending retrograde from the left CFA across the aortic bifurcation into the right CFA through very tortuous iliac arteries. The operator was unable to advance a sheath through these tortuous vessels, (b) Sagittal maximal intensity projection reconstruction computed tomography angiogram of the right groin demonstrating nearly 10 cm of subcutaneous tissue from the skin to the common femoral artery via antegrade access at 60°, (c) Sagittal maximal intensity projection reconstruction computed tomography angiogram of the right groin showing only 5.5 cm of subcutaneous tissue from the skin to the superficial femoral artery at 60°.
MATERIALS AND METHODS
This study was granted an exemption by our institutional review board due to the retrospective nature of data collection. A retrospective review of CPT codes was performed to identify all patients undergoing angiography in a single practice between May 2011 and January 2015. All patients with antegrade SFA access and an Angio-Seal hemostatic closure device (St. Jude Medical, St. Paul, MN, USA) were included in this study. Patients with arterial access other than antegrade SFA were excluded. All antegrade SFA arteriotomies were closed with Angio-Seal, and no other closure device was used. There were no patients with a history of a surgical procedure or bypass at the access site.
Patient sample
A total of 140 ultrasound-guided antegrade SFA punctures with subsequent Angio-Seal closure were performed in 110 limbs in 88 unique patients (Table 1). 65.9% of patients had one procedure, 18.2% had two procedures, 10.2% had three procedures, 3.4% had four procedures, 1.1% had five procedures, and 1.1% had six procedures. 29 (20.7%) procedures were performed in women, and 111 (79.3%) were performed in men. The indication for the majority of procedures (120/140) was non-healing ulcer disease, Fontaine Stage IV.[7,8] In addition, 6 procedures were for rest pain (Fontaine Stage III), 11 procedures were for claudication (Fontaine Stage II), 2 for frostbite, and one for treatment of a vascular malformation (Fontaine stage not applicable). Patient age, sex, body mass index (BMI), Fontaine stage, sheath size, and intraoperative heparin and protamine doses were recorded, and a thorough chart review was conducted to screen for complications. SFA diameter was directly measured on saved ultrasound and computed tomography (CT) images if available; for the few cases in which these were unavailable, diameter was measured on digital subtraction angiography with magnification correction. Arterial sheath sizes ranged from 5-F to 10-F with the vast majority (124/140) between 5-F and 6-F. The 8-F Angio-Seal device was used in one case; the 6-F Angio-Seal device was used in all others.
| Mean age±SD | 69.2±10.9 |
| Male sex | 111 (79%) |
| Fontaine stage | |
| II | 11 (8%) |
| III | 6 (4 %) |
| IV | 120 (86%) |
| n/a | 3 (2%) |
| Number of procedures per patient | |
| 1 | 58 (66%) |
| 2 | 16 (18%) |
| 3 | 9 (10%) |
| 4 | 3 (3%) |
| 5 | 1 (1%) |
| 6 | 1 (1%) |
SD: Standard deviation, SFA: Superficial femoral artery
Technique
Two operators D.M. and P.K. performed all of the antegrade punctures and closures in the interventional radiology (IR) suite. Following local anesthesia with lidocaine, the SFA was punctured under ultrasound guidance with a micropuncture needle kit. Ultrasound was used to avoid plaques and select an optimal landing zone for Angio-Seal deployment. Diagnostic angiography was performed with a 5-F or 6-F introducer sheath. All patients undergoing intervention were treated with IV heparin to maintain activated coagulation time (ACT) above 250. Three patients were reversed with protamine for a high ACT before closure. Operator D.M. did not recheck an ACT before closure on any patient. After closure with the Angio-Seal device, the site was immediately checked by the physician, evaluating pedal and femoral pulses distal to the access site. 5–10 min after each Angio-Seal deployment, the groin site was interrogated by the IR nursing staff for possible hematoma. A sterile dressing was then placed at the access site, and the patient was monitored in the radiology care unit. All patients were on bed rest with straight leg orders for 2 h and were subsequently discharged home after tolerating ambulation with assistance. The groin site was checked by nursing before departure.
Follow-up
In addition to immediate post-procedural follow-up discussed above, all patients were seen in clinical follow-up at the IR clinic at intervals of 1 and 6 months; follow-up imaging was not routinely obtained.
Study endpoints
Technical success was defined as successful deployment of the Angio-Seal with immediate achievement of hemostasis and preserved distal pulses. Complications were recorded and classified by the society of IR A-F system.[9]
Statistical analysis
Fisher’s exact test was used to compare complication rates between different procedure and patient characteristics. Fisher’s exact test was also used to compare complication rates between different SFA diameter sizes within the female patient group. A statistically significant difference in complication rates between groups was defined as a p < 0.05. Intraoperative heparin doses were unavailable for five procedures in 4 patients, none with complications; these cases were omitted from statistical analysis of heparin doses but were included for the remainder of the calculations.
RESULTS
Technical success rate (n = 140): 136 (97.1%).
Complication rates per procedure:
Any complication: 11 (7.9%).
Minor (Grades A and B): 8 (5.7%).
Major (Grades C and D): 3 (2.1%).
Technical success was achieved in 136/140 procedures (97.1%). Complications were noted in 11 cases in 11 patients, 4 of which involved technical failure of Angio-Seal deployment. Of the 4 failed closures, 2 were due to unsuccessful achievement of hemostasis after device deployment, requiring manual compression which was ultimately successful. One of these two patients developed a delayed groin hematoma recognized clinically 5 days later, requiring blood transfusion, and a 5-day hospitalization (Grade D). The other patient developed a delayed complete occlusion of the SFA at the access site 3 weeks later that was recognized and successfully angioplastied and stented, but not before causing exacerbation of pre-existing ischemic toe gangrene ultimately requiring amputation of two toes (Grade D). The other two technical Angio-Seal deployment failures were due to immediately recognized eccentric stenoses at the SFA access site, likely related to the collagen plug projecting into the arterial lumen, or spontaneous thrombus formation due to the footplate (Figure 2). One was detected incidentally when a femoral arteriogram was performed immediately through existing contralateral retrograde CFA access; the other was suspected based on diminished distal pulses in the ipsilateral foot, prompting further evaluation through contralateral retrograde CFA access. In both of these cases, the stenoses were identified before the patient left the angiography suite and were successfully and immediately treated with angioplasty and stent grafting. No hospitalization was required (Grade B).
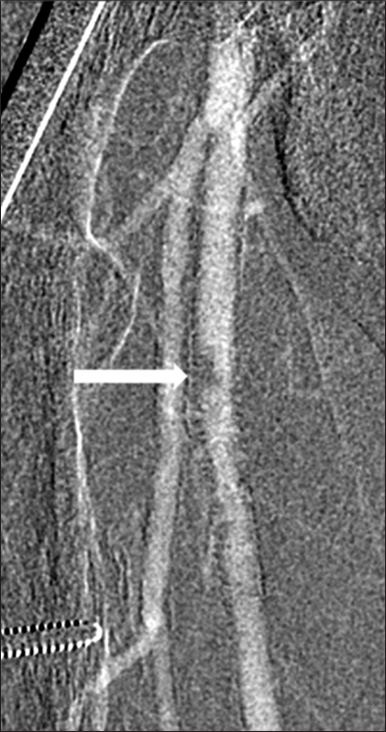
- 63-year-old man with chronic kidney disease and atherosclerosis presenting with non-healing right toe ulcers and rest pain due to infrapopliteal arterial occlusive disease, who underwent endovascular revascularization via antegrade SFA access with Angio-Seal closure. Carbon dioxide digital subtraction angiography of the right thigh immediately after Angio-Seal deployment demonstrates a discrete ovoid eccentric filling defect in the proximal right SFA at the earlier puncture site.
There were two self-limited groin hematomas requiring no therapy (Grade A): One was discovered on physical examination 13 days post-procedure and confirmed by ultrasound. One occurred spontaneously due to oozing around the sheath before its removal in a patient who had undergone 48 h of catheter-directed TPA thrombolysis via the antegrade SFA access. This latter hematoma was deemed unrelated to Angio-Seal closure.
3 SFA access site pseudoaneurysms occurred, with maximal diameters of 1.9, 3.3, and 3.5 cm, respectively. 2 were detected within 1–3 days on physical examination, and one was detected incidentally on ultrasound 45 days later. All were successfully treated with percutaneous thrombin injection (Grade B).
There were 2 cases of delayed SFA access site stenosis due to eccentric filling defects likely related to the collagen plug and/or reaction to the footplate. In one case, the stenosis was discovered incidentally on subsequent angiography that was performed for the treatment of severe anterior tibial and posterior tibial occlusive disease. The stenosis was treated with a covered stent and no hospitalization was required (Grade B). The other case of delayed access site stenosis occurred in a patient with pre-existing foot ulcers due to severe anterior tibial occlusive disease. The left SFA was accessed antegrade for angiography twice within 5 days, but neither procedure was successful in crossing or treating the anterior tibial occlusion. Delayed eccentric SFA stenosis (presumably related to the Angio-Seal plug and/or footplate) was detected on CT angiography 17 months after the initial SFA access. This patient was then brought back for repeat angiography, and the lesion was treated with angioplasty and stenting (Figure 3). As her pre-existing foot ulceration had worsened, she underwent transmetatarsal amputation (Grade D). 7 months later, she received a below-knee amputation after she developed osteomyelitis and recurrent ulceration unrelated to the prior SFA lesion.
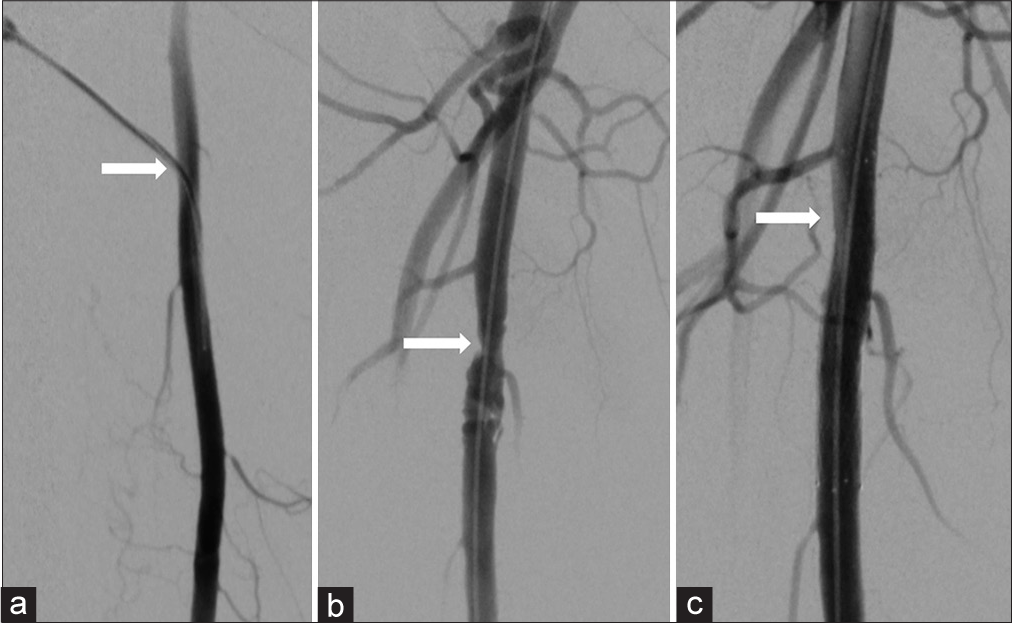
- (a) 52-year-old woman presenting with non-healing right foot ulcers due to infrapopliteal arterial occlusive disease, who underwent endovascular revascularization via antegrade SFA access with Angio-Seal closure. Her symptoms later recurred, and she returned for repeat angiography 17 months later. Digital subtraction angiography of the right thigh demonstrating a 5-french sheath (arrow) entering the widely patent proximal right SFA during her original procedure, (b) Digital subtraction angiography of the right thigh 17 months later demonstrating irregular stenosis (arrow) at the prior superficial femoral artery puncture site, (c) Magnified digital subtraction angiography of the right thigh following angioplasty and stenting shows no significant residual stenosis.
Angio-Seal complication rates compared by risk factor categories are provided in (Table 2). Complication rates for each vessel diameter within the female patient subgroup are provided in (Table 3).
| Risk factor | Complication rate n, (%) | P |
|---|---|---|
| Heparin dose* | ||
| <5000 u | 1/16 (6.25) | 0.183 |
| 5000 u | 7/53 (13.2) | |
| >5000 u | 3/66 (4.5) | |
| Sheath size | ||
| 5 fr | 3/20 (15) | 0.377 |
| 6 fr | 7/104 (6.7) | |
| 7-10 fr | 1/16 (6.3) | |
| SFA diameter | ||
| <7 mm | 1/34 (2.9) | 0.527 |
| 7 mm | 6/59 (10.2) | |
| >7 mm | 4/47 (8.5) | |
| BMI | ||
| <25 | 4/38 (10.5) | 0.675 |
| ≥25, <30 | 4/47 (8.5) | |
| ≥30 | 3/55 (5.5) | |
| Protamine given | 0/3 (0) | |
| No protamine given | 11/137 (8) | 1.0 |
| Age | ||
| <60 | 1/27 (3.7) | |
| 60-69 | 5/40 (12.5) | 0.402 |
| 70-79 | 2/47 (4.3) | |
| ≥80 | 3/26 (11.5) | |
| Fontaine stage | ||
| None | 1/3 (33) | |
| II | 1/11 (9.1) | 0.180 |
| III | 1/6 (16.7) | |
| IV | 8/120 (6.7) | |
| Sex | ||
| Male | 5/106 (4.5) (3 hematoma/pseudoaneurysm, 2 SFA stenosis) | 0.0105 |
| Female | 6/23 (20.7) (3 hematoma/pseudoaneurysm, 3 SFA stenosis/occlusion) | |
*Heparin dose was unavailable for 5 procedures performed in 4 different patients, none of whom had complications. These cases were omitted from statistical analysis of heparin doses but were included in the other analyses. SFA: Superficial femoral artery, BMI: Body mass index
| SFA diameter | Complication rate n, (%) | P |
|---|---|---|
| 5 mm | 0/4 (0) | 0.361 |
| 6 mm | 1/9 (11.1) | |
| 7 mm | 4/10 (28.6) | |
| 8 mm | 1/2 (50) |
SFA: Superficial femoral artery
DISCUSSION
PAD is a growing public health issue that is estimated to affect approximately 8 million Americans and is associated with significant morbidity and mortality.[10] Among Americans 65 and older, an estimated 12%–20% are affected.[11] Angiography and endovascular revascularization are accepted first-line treatments for the vast majority of patients with debilitating PAD.[12-14] Conventionally, access for endovascular revascularization has been the contralateral CFA with an “up and over” approach. The inherent challenges of contralateral access have led to the exploration of a multitude of alternative access sites and access site techniques.[15-18] Antegrade access is an increasingly common approach to avoid the challenges of contralateral access.[1]
The most commonly described site for antegrade access for lower extremity intervention has been the ipsilateral CFA. However, there are documented limitations and disadvantages of antegrade CFA access. Proximity of the access site can lead to time-consuming selection of the SFA, especially in the obese patient.[2] Barriers to antegrade CFA puncture also include the “hostile groin” (scar tissue, aneurysmal CFA, groin infection, hip flexion deformity, and previously failed CFA punctures).[19] Gutzeit et al. proposed a possible time advantage of antegrade SFA access over CFA access with a mean access time of 3.5 min in 98 patients with US-guided SFA access.[4] The 3.5 min US-guided antegrade SFA access time is less than half previously reported mean times associated with retrograde (8.3 min) and antegrade (8.0 min) CFA approaches.[3] Marcus et al. concluded similar benefit to ultrasound-guided antegrade SFA puncture versus CFA puncture in 30 patients with hostile groins.[19] Decreased screen time, radiation doses, and complications were noted in the antegrade SFA access versus CFA. Similar safety and efficacy of antegrade SFA punctures were demonstrated by Kweon et al. with 100% technical success and one minor hematoma in 28 patients.[6]
There are very little data on the safety and efficacy of the Angio-Seal closure device in antegrade SFA access. There have been several studies evaluating Angio-Seal closure in antegrade access, but the majority of access sites were CFA, not SFA.[1,5,20-22] Gutzeit et al. investigated Angio-Seal closure of antegrade access of both the SFA and CFA and reported a complication rate of 8.9% in 178 cases, similar to our experience.[5] Nearly all of these complications were pseudoaneurysms or hematomas, with only one vascular occlusion. Mukhopadhyay et al. experienced only 1 minor groin hematoma and 1 episode of worsening lower limb ischemia in 21 patients with Angio-Seal closure of antegrade CFA groin punctures.[20] Lupattelli et al. described low overall complication rates (<2.5%) associated with 1889 Angio-Seal closures of antegrade CFA punctures but did not investigate SFA punctures.[1] These were similar to the complication rates for retrograde Angio-Seal and manual compression. The success rate of hemostatis after Angio-Seal closure for antegrade CFA puncture was 97.9%. Katzenschlager et al. reported 2 small hematomas and 1 pseudoaneurysm in 105 patients with Angio-Seal closure of the CFA. There was no association with “high-risk” punctures including 69 antegrade punctures, 22 obese, and 32 hypertensive patients.[21] More recently, Chaudhuri et al. compared Angio-Seal closure in patients undergoing antegrade versus retrograde CFA access and reported a success rate of 91% in 271 antegrade cases versus a 96.6% in 237 retrograde cases.[22]
The overall complication rate (7.8%) and technical success rate (97.1%) in this study are on par with previously reported case series and support the growing body of evidence that Angio-Seal closure for antegrade SFA access is efficacious and safe. Obesity, female sex, and advanced age have been described as risk factors for increased vascular complications previously.[2,23-25] This study did not identify any statistically significant increase in complications related to age, BMI, intraoperative heparin dose, sheath size, or Fontaine stage.
We did identify a significant difference in the complication rate between male (4.5%) and female (20.7%) patients (p = 0.01). Significantly increased complication rates associated with collagen-based vascular closure devices (VCDs) in female patients have previously been reported and thought to relate at least in part to smaller arterial diameter.[26-28] However, our data did not show an increase in complication rates with smaller vessel diameter, neither within the total group nor within the female subgroup. In fact, nearly all (5/6) of the female patients with complications had an SFA diameter of 7 mm or greater, comparable to the male average diameter. The fact that this study shows more frequent complications in females despite a lack of correlation with vessel size suggests that additional factors may be involved, and further investigation is warranted. The severity of complications did not differ significantly between males and females, with a spectrum from minor hematomas to pseudoaneurysms to SFA stenosis or occlusion all occurring in both groups.
This study only sought to evaluate the Angio-Seal VCD, as this was the VCD with which the authors had the most prior experience. Several other VCDs are currently available for arteriotomy closure, however, and may offer different advantages. Given that the most serious complications observed in this study related to stenosis or occlusion of the access site following Angio-Seal deployment, an attractive option would be the Mynx VCD (Cordis, Milpitas, CA), which is designed to achieve hemostasis with a polyethylene glycol sealant which expands outside of the artery, theoretically leaving nothing inside the vessel and eventually becoming hydrolized. However, despite the extravascular design of the Mynx VCD, there have been reports of intravascular complications. Fields et al.[29] reported five cases (18% of 27 studies) of intravascular Mynx sealant identified on follow-up angiography or sonography after VCD deployment, one of which resulted in symptomatic SFA stenosis requiring surgical excision of the sealant, and pathologic examination confirmed that the offending lesion was indeed Mynx sealant. Islam et al.[30] reported a case of limb ischemia due to popliteal artery embolization with Mynx sealant following VCD deployment in the CFA. Nonetheless, a larger study[31] comparing Angio-Seal to Mynx closure of CFA arteriotomies has demonstrated similarly low major complication rates (2.1% of 190 Angio-Seal patients vs. 2.1% of 238 Mynx patients) between the two devices, and future investigation to compare efficacy and complication rates in SFA closure may be warranted.
This study is limited by its retrospective nature and lack of a control group. In addition, as post-procedural patient follow-up relied primarily on clinical examination and patient symptoms rather than early scheduled imaging, subclinical complications such as early asymptomatic collagen plug-related stenosis or small hematomas might have been missed. In particular, cases of initially occult plug dehiscence might be detected much earlier by non-invasive ultrasound, allowing for treatment before patients deteriorate clinically. This could also help improve understanding of the natural history of delayed plug and footplate-related complications. The authors are aware anecdotally that some practices routinely obtain repeat non-invasives as soon as the day following a peripheral arterial intervention. Our data suggest that early post-procedural duplex imaging may be valuable in patients, particularly women, who undergo closure of antegrade SFA access.
CONCLUSIONS
In patients for whom CFA access is not readily achievable or ideal, antegrade superficial femoral access provides an important conduit for endovascular treatment of distal lower extremity arterial disease. The Angio-Seal VCD is a safe and effective method of arteriotomy closure for antegrade arterial access in patients undergoing lower extremity endovascular therapy. An increased rate of complications in female patients warrants cautious selection of appropriate patients and may compel early post-procedural follow-up imaging.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Efficacy and safety of antegrade common femoral artery access closure using the angio-seal device: Experience with 1889 interventions for critical limb ischemia in diabetic patients. J Endovasc Ther. 2010;17:366-75.
- [CrossRef] [PubMed] [Google Scholar]
- Obesity: An independent risk factor for insufficient hemostasis using the angioSeal vascular closure device after antegrade puncture. Cardiovasc Intervent Radiol. 2012;35:775-8.
- [CrossRef] [PubMed] [Google Scholar]
- Retrograde vs. Antegrade puncture for infra-inguinal angioplasty. Cardiovasc Intervent Radiol. 2003;26:370-4.
- [CrossRef] [PubMed] [Google Scholar]
- Antegrade access to the superficial femoral artery with ultrasound guidance: Feasibility and safety. J Vasc Interv Radiol. 2010;21:1495-500.
- [CrossRef] [PubMed] [Google Scholar]
- Feasibility and safety of vascular closure devices in an antegrade approach to either the common femoral artery or the superficial femoral artery. Cardiovasc Intervent Radiol. 2012;35:1036-40.
- [CrossRef] [PubMed] [Google Scholar]
- Antegrade superficial femoral artery versus common femoral artery punctures for infrainguinal occlusive disease. J Vasc Interv Radiol. 2012;23:1160-4.
- [CrossRef] [PubMed] [Google Scholar]
- Surgical treatment of peripheral circulation disorders. Helv Chir Acta. 1954;21:499-533.
- [Google Scholar]
- Recommended standards for reports dealing with lower extremity ischemia: Revised version. J Vasc Surg. 1997;26:517-38.
- [CrossRef] [Google Scholar]
- Quality improvement guidelines for the reporting and archiving of interventional radiology procedures. J Vasc Interv Radiol. 2003;14:S293-5.
- [CrossRef] [Google Scholar]
- Heart disease and stroke statistics--2011 update: A report from the american heart association. Circulation. 2011;123:e18-e209.
- [CrossRef] [Google Scholar]
- Prevalence of peripheral arterial disease and risk factors in persons aged 60 and older: Data from the national health and nutrition examination survey 1999-2004. J Am Geriatr Soc. 2007;55:583-9.
- [PubMed] [Google Scholar]
- Bypass versus angioplasty in severe ischaemia of the leg (BASIL): Multicentre, randomised controlled trial. Lancet. 2005;366:1925-34.
- [CrossRef] [Google Scholar]
- Shifting paradigms in the treatment of lower extremity vascular disease: A report of 1000 percutaneous interventions. Ann Surg. 2007;246:415-22.
- [CrossRef] [PubMed] [Google Scholar]
- Contemporary results of angioplasty-based infrainguinal percutaneous interventions. J Vasc Surg. 2005;42:932-9.
- [CrossRef] [PubMed] [Google Scholar]
- Retrograde pedal/tibial artery access for treatment of infragenicular arterial occlusive disease. Methodist Debakey Cardiovasc J. 2013;9:73-8.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous retrograde tibial access in limb salvage. J Endovasc Ther. 2003;10:614-8.
- [CrossRef] [PubMed] [Google Scholar]
- Retrograde popliteal approach for challenging occlusions of the femoral-popliteal arteries. J Vasc Surg. 2013;58:84-9.
- [CrossRef] [PubMed] [Google Scholar]
- Retrograde pedal access for patients with critical limb ischemia. J Vasc Surg. 2014;60:375-81.
- [CrossRef] [PubMed] [Google Scholar]
- Access to the superficial femoral artery in the presence of a “hostile groin”: A prospective study. Cardiovasc Intervent Radiol. 2007;30:351-4.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and complications of angioseal in antegrade puncture. Eur J Radiol. 2005;56:409-12.
- [CrossRef] [PubMed] [Google Scholar]
- Angio-seal use in patients with peripheral arterial disease (ASPIRE) Angiology. 2009;60:536-8.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous vascular closure using an anchored collagen plug provides effective haemostasis following both antegrade and retrograde femoral arterial punctures. Eur J Vasc Endovasc Surg. 2014;48:220-5.
- [CrossRef] [PubMed] [Google Scholar]
- Quality improvement guidelines for vascular access and closure device use. J Vasc Interv Radiol. 2014;25:73-84.
- [CrossRef] [PubMed] [Google Scholar]
- Vascular closure devices: The second decade. J Am Coll Cardiol. 2007;50:1617-26.
- [CrossRef] [PubMed] [Google Scholar]
- Risk of local adverse events following cardiac catheterization by hemostasis device use and gender. J Invasive Cardiol. 2004;16:459-64.
- [Google Scholar]
- Impact of gender on femoral access complications secondary to application of a collagen-based vascular closure device. J Invasive Cardiol. 2004;16:247-50.
- [Google Scholar]
- Acute leg ischemia: The dark side of a percutaneous femoral artery closure device. Ann Vasc Surg. 2006;20:278-81.
- [CrossRef] [PubMed] [Google Scholar]
- Complications associated with the angio-seal arterial puncture closing device: Intra-arterial deployment and occlusion by dissected plaque. J Vasc Surg. 2006;44:1357-9.
- [CrossRef] [PubMed] [Google Scholar]
- Femoral artery complications associated with the mynx closure device. AJNR Am J Neuroradiol. 2010;31:1737-40.
- [CrossRef] [PubMed] [Google Scholar]
- Popliteal artery embolization with the mynx closure device. Catheter Cardiovasc Interv. 2010;75:35-7.
- [CrossRef] [PubMed] [Google Scholar]
- Vascular complications after percutaneous coronary intervention following hemostasis with the mynx vascular closure device versus the angioSeal vascular closure device. J Invasive Cardiol. 2010;22:175-8.
- [Google Scholar]







