Translate this page into:
Imaging-guided Percutaneous Large-Bore Suprapubic Cystostomy, a Safe Bladder Diversion Alternative

Corresponding Author: Jesse Chen, Department of Radiology, Staten Island University Hospital, 475 Seaview Avenue, Staten Island, NY, 10305, United States. E-mail: jessechen10@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Chen J, Landau E, Ahmad N, Giordano C, Scheiner J, Katlowitz N, Geller D. Imaging-guided Percutaneous Large-Bore Suprapubic Cystostomy, a Safe Bladder Diversion Alternative. Am J Interv Radiol 2018; 2(10) 1-6.
Abstract
Suprapubic catheter placement is a common method of bladder diversion. To date, there are limited reports describing safe placement of large-bore (18–28 F) catheters as smaller catheters often clog and require upsizing procedures. We retrospectively evaluate the image-guided percutaneous placement of large-bore catheters by interventional radiologists in our institution, totaling 51 catheters in 51 patients over 5 years. We successfully placed a large-bore catheter in 96% (49/51) of first attempts with no post-procedural complications. This data demonstrates that percutaneous placement of large-bore suprapubic catheters by interventional radiologists is a safe and less-invasive bladder diversion alternative to traditional blind or open surgical techniques.
Keywords
Cystostomy
Large bore
Suprapubic
INTRODUCTION
Suprapubic urinary bladder catheters (SPCs) play an important role in patients requiring long-term bladder catheterization and/or diversion. Large Cochrane review analyses have demonstrated that the use of SPC compared to transurethral catheterization decreases bacteriuria, need for recatheterization, and discomfort.[1] Patients with transurethral catheters have been shown to have significantly more hospital visits compared to SPC patients due to pain.[2] Equally important, patient preference of an SPC over a transurethral catheter is also documented due to its ease of management, decreased relative discomfort, and decreased rate of infections.[3]
In the majority of cases, the distended bladder displaces the peritoneal reflection superiorly allowing for uneventful “blind” insertion of such catheters. However, there is a reported 2.4% incidence of bowel injury with a “blind” technique, and even under cystoscopic guidance, a 30-day mortality rate of 1.8% is reported.[3] Certain patient risk factors increase the risk for complications including obesity, history of radical pelvic surgery, and a short distance (≤11 cm) between the symphysis pubis and umbilicus.[4] The last line alternative of open surgical placement is a less favorable option as it is more invasive, requires general anesthesia, and carries further post-operative risk and cost.
Placement of relatively small SPC (10–14 French) is commonly performed in the emergent setting for acute urinary retention when urethral catheterization is either impossible or contraindicated. The use of ultrasound assistance has been described in this emergency setting, including one series of 17 patients with 100% success rate.[5] However, these catheters are not optimal for prolonged, chronic drainage. Placement of a large-bore catheter on initial cystostomy could preclude the need for repeat procedures for catheter upsizing.
Initially described in 1989, ultrasound- and fluoroscopic-guided placement of large-bore SPC (18 French or larger) by interventional radiologists has been shown to be a safe and effective alternative insertion method without requiring general anesthesia.[6] In our study, we describe a single institution’s experience placing large-bore SPC (18–28 French) within the interventional radiology (IR) department including the technical success rate and complications.
MATERIALS AND METHODS
Approval for this retrospective review was obtained from the hospital’s Institutional Review Board. The study was HIPAA compliant and informed consent was waived.
Patient data collection
Our institution’s dictation database was searched for all suprapubic catheter insertions performed by the department of IR over a 5-year period from January 1, 2012, to December 31, 2016. A total of 51 consecutive primary large-bore SPC insertions were performed. The electronic medical record for these patients was reviewed to abstract data regarding gender, age, indication, patient comorbidities, catheter diameter, anesthetic provided, and both intraoperative and subsequent in-house complications.
Technique
All procedures were done by one of four full-time attending staff interventional radiologists ranging from 1 to 20 years of experience. The first 5 cases were performed conjointly between the IR and urology departments. Once comfortable with the technique, IR performed all subsequent procedures without assistance.
Our institutional Picture Archiving and Communication System were reviewed before all insertions for any cross-sectional imaging that would demonstrate pelvic anatomy and a safe window for percutaneous insertion. Once in the IR suite, an initial ultrasound was performed to evaluate for bladder distention. If not already distended, the urinary bladder was filled with 150–300 cc normal saline through a pre-existing transurethral catheter to approximate the bladder and anterior abdominal wall and to displace interposing bowel loops. A safe window was confirmed through a combination of ultrasound, fluoroscopy, and on occasion, pre-procedural CT.
A 5 French, 7 cm needle/trocar assembly (Yueh needle, Cook Medical, Bloomington, IN) was subsequently advanced into the bladder under ultrasound guidance. Alternatively, a 21 g micropuncture set was used. Once urine was aspirated back, a small volume of diluted contrast was administered through the outer sheath to confirm intravesicular positioning under fluoroscopic guidance (Figure 1a). The inner stylet was removed and a 0.035-inch Amplatz guidewire (Boston Scientific, Marlborough, MA) was advanced into the bladder under fluoroscopic guidance (Figure 1b). The Yueh sheath was removed and the tract was serially dilated to advance a 10 mm, 15 cm balloon catheter system (Bard, Covington, GA) which ultimately dilated the subcutaneous tract and cystostomy to 30 French under fluoroscopic control (Figure 1c). A 30F peel-away sheath was advanced over the balloon, the balloon was deflated and removed, and a large-bore Foley catheter (usually 26 French) was directed into the urinary bladder through the peel-away sheath (Figure 1d). With the sheath then removed, a small amount of diluted contrast was administered through the catheter to confirm placement. The retention balloon was then distended with sterile water under fluoroscopic control.
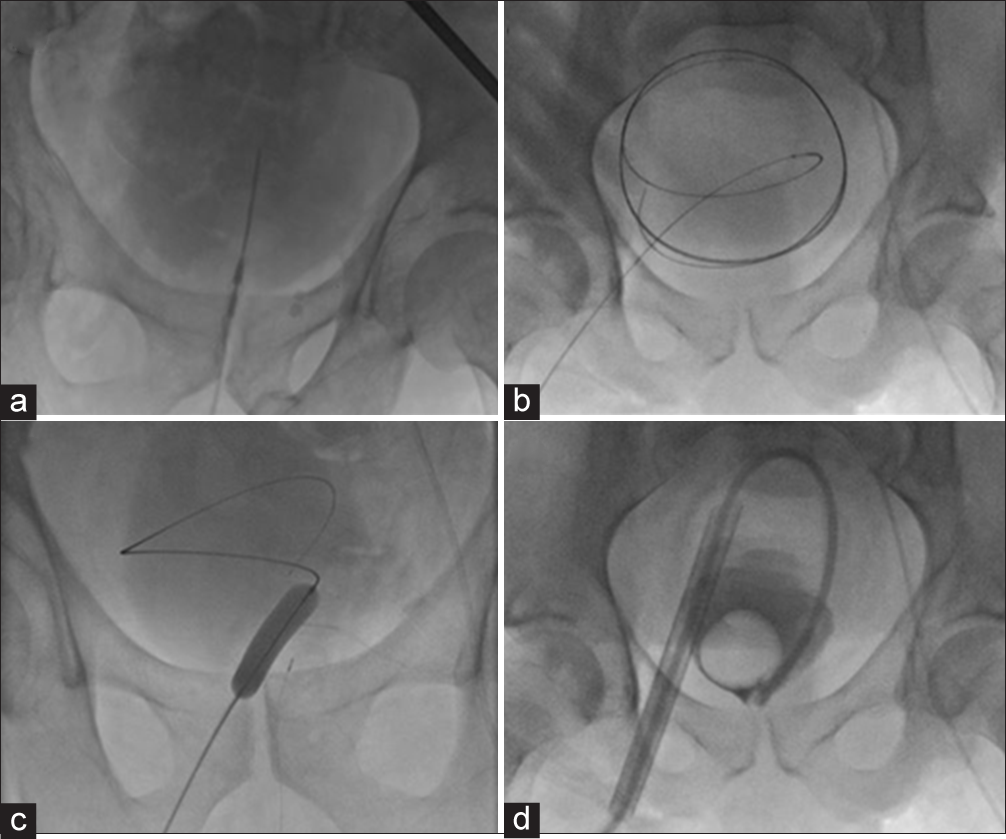
- A 70-year-old male with urinary retention from BPH undergoing percutaneous image-guided suprapubic catheterization. (a) Fluoroscopic appearance of a 7 cm Yueh needle within the distended bladder. Opacification of the bladder following injection of a small amount of Cysto-Conray contrast confirms needle tip position. (b) Fluoroscopic appearance of a 0.035-inch Amplatz guidewire threaded into the bladder, with expected coiled appearance. (c) Fluoroscopic appearance of a 30F balloon passed over the guidewire to dilate the cystostomy. (d) Fluoroscopic appearance of a 30F peel-away sheath within the bladder. A large-bore catheter is advanced through the sheath and its retention balloon inflated.
Follow-up of patients was limited to the same admission for inpatient procedures and a follow-up phone call within 1 week of discharge for outpatient procedures.
RESULTS
Patient demographics
From January 2012 to December 2016, 51 large-bore suprapubic catheters were inserted in 51 patients (46 males, 5 females) among them 49 of 51 were single stage procedure. The mean age was 67.3 years, ranging from 22 to 92. 10 cases were performed as outpatient procedures, and the remaining 41 cases were performed as inpatient procedures. One case was performed with general anesthesia due to patient preference. The indications for suprapubic catheterization are listed in Table 1.
| Indication | Count |
|---|---|
| Neurogenic bladder (stroke, spinal trauma) | 16 |
| BPH w/urinary retention | 12 |
| Urethral stricture/injury | 8 |
| Penile trauma | 6 |
| Fournier’s Gangrene/necrotizing fasciitis | 3 |
| Vulvar cancer | 2 |
| Prostate cancer | 1 |
| Hypospadias | 1 |
| Bladder injury during bowel surgery | 1 |
| Chronic urinary calculi | 1 |
| Total | 51 |
Outcomes
Technical success was defined as single-step placement of the SPC in the bladder as planned. Of the 51 cases performed, technical success was achieved in 49 procedures, for a 96% success rate. No major complications occurred according to the Society of IR Clinical Practice Guidelines.[7] For the first unsuccessful case, there was immediate clinical suspicion of the catheter being malpositioned outside of the urinary bladder on completion of the procedure. The patient was taken directly to computed tomographic (CT), and a CT cystogram confirmed the catheter was positioned within the space of Retzius (Figure 2). The patient was subsequently taken back to the IR suite for uneventful removal of the malpositioned catheter and successful placement of a new 26F SPC.
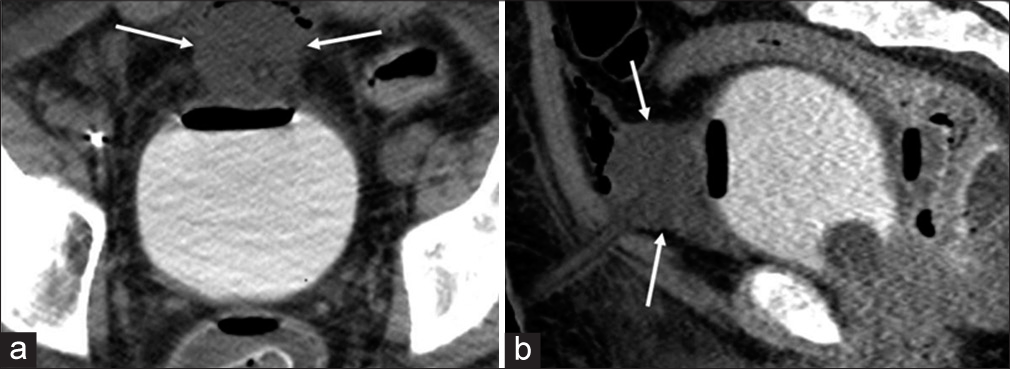
- A 37-year-old male with diffuse genital burn injury following motor vehicle collision underwent suprapubic catheterization. (a) Axial and (b) sagittal postprocedural computed tomographic cystogram images demonstrate the inflated Foley catheter balloon in the space of Retzius (white arrows)
In the second unsuccessful case, the bladder was accessed uneventfully. However, after balloon dilatation of the abdominal wall tract, the 30-French trocar sheath was unable to be advanced into the bladder. After several attempts to advance the sheath, extra-luminal contrast extravasation increased so a 14-French pigtail drainage catheter was easily placed instead. The patient was brought back the next day for uneventful catheter upsizing to a 26F catheter.
The distribution of catheter sizes placed on the first attempt is listed in Table 2. There were no in-house complications for the 41 inpatient procedures. Four of our patients subsequently expired in the same admission: Two from sepsis in the setting of Fournier’s gangrene, one from sepsis from overwhelming bed sores, and one from withdrawal of care in the setting of prolonged ventilator dependence after diffuse burn injury. The placement of an SPC, however, had no identifiable contributory role to the cause of death for any patients. On telephone follow-up for outpatient procedures, there were no reported complications. Long-term follow-up for these patients was in urology clinic.
| Initial catheter size | Count |
|---|---|
| 18 | 1 |
| 20 | 1 |
| 22 | 2 |
| 26 | 41 |
| 28 | 4 |
| Total | 49 |
DISCUSSION
Suprapubic catheters are commonly used for both temporary and long-term urinary drainage. Suprapubic cystostomy is indicated when transurethral catheterization is contraindicated or technically impossible. The traditional approach of suprapubic catheterization is with an open abdominal incision under general anesthesia. Some urologists prefer this method as it provides reassurance that no bowel is harmed during the procedure. However, the invasiveness and length of the surgery, the post-procedural pain, the need for anesthesia, and the cost to both the patient and the hospital are all reasons to pursue a safe and efficient alternative.
While percutaneous placement is technically simple, the risk for visceral injury is not excluded from the study. Ahluwalia et al. reported a 10% intraoperative complication rate, 2.4% risk of bowel injury, 19% 30-day complication rate following a blind technique, and a 1.8% mortality rate in 219 urology patients who underwent percutaneous suprapubic insertion with cystoscopic guidance.[3] Despite the possibility of procedural complications, suprapubic catheterization is preferred by 89% of patients over urethral catheterization mostly based on comfort and ease of use, while providing a decreased risk of infection.[3] One prospective review of men catheterized either transurethrally or suprapubically for prostatic enlargement reported a 3-year incidence of urinary tract infection of 40% in the transurethral group and 18% in the suprapubic group.[8]
There are a number of complications that may arise from SPC. Spontaneous intravesical catheter knotting has been reported although a described risk factor for this complication is smaller catheter diameter.[9,10] Migration of an 18F SPC into a ureter resulting in obstruction and pyelonephritis has been described; however, this complication might be avoided with larger catheters.[11] Incisional hernia following SPC insertion is described although this complication is rare.[12] Leakage around an SPC is also a possible complication, however, not unique to SPC.
Practice guidelines published by the British Association of Urological Surgeons (BAUS) recommend considering SPC in all patients with chronic urinary retention, neurological disease, urinary incontinence, post-operative care needs, traumatic injury, and those with palliative needs.[13] Ultrasound has been recommended by the BAUS as an adjunct to SPC insertion to ensure that no interposing bowel loops are present. However, the society warns that “only individuals who have received specific training and are experienced with this task” should utilize this technology. Although interventional radiologists are specially trained in ultrasound- and fluoroscopic-guided catheter placement, very few published studies describe the role the interventionalist plays regarding SPC. This retrospective case series presents the safe placement of large-bore catheters, ranging from 18 to 28F, by a small community-based IR department in 51 patients.
Large-bore suprapubic catheter placement under combined ultrasound and fluoroscopic guidance has proven to be safe and efficient. There were no serious intraoperative complications, no complications observed during the same hospital admission, and two-step procedure was rarely necessary (2 out of 51). Using direct real-time imaging, interposed bowel can be avoided, and percutaneous placement can be achieved, both in obese patients and those with complex post-operative abdominal/pelvic walls or otherwise abnormal anatomy.
Two cases we performed recently reinforce the importance of imaging guidance. These patients were not included in the above series because the first was a planned two-step procedure and the second was performed more recently, outside of the data collection window.
An 83-year-old male with neurogenic bladder had a preprocedural pelvic CT demonstrating a bowel loop traversing the space of Retzius (Figure 3), and it was decided an initial 14F SPC would be placed under CT guidance. The patient was subsequently brought to the IR suite for exchange of the 14F pigtail for a 26F Foley. Given the previously mentioned 2.4% chance of bowel injury in traditional SPC insertion, any effort to avoid intervening bowel is critical. This case demonstrates how interventional radiologists, familiar with performing procedures under imaging guidance, may be able to safely place an SPC even when there is a narrow percutaneous window, or when the bladder cannot be ideally opposed to the ventral abdominal wall (e.g. adhesions and prior surgery).
 Figure 3
Figure 3- An 83-year-old male with neurogenic bladder was planned for suprapubic catheterization. (a) Axial and (b) sagittal preprocedural abdominopelvic computed tomography demonstrates a loop of bowel traversing the anterior bladder (white arrows), narrowing the window across which a percutaneous suprapubic urinary bladder catheter may be inserted safely.
During SPC placement in a 74-year-old male with neurogenic bladder, the final image taken to confirm catheter placement demonstrated that the contrast injected into the bladder collected in a limited ring around the Foley balloon (Figure 4a). Lateral fluoroscopic imaging showed the contrast limited to the posterior pelvis, without inferior extension into the retropubic space as would be expected (Figure 4b). Intra-procedural re-evaluation of a recent abdominal CT confirmed the presence of a posterior bladder diverticulum (Figure 4c). On deflation of the Foley balloon, the contrast flowed freely into the retropubic space (Figure 4d). The Foley was retracted 5 cm and the balloon was reinflated. Without the ability to evaluate the patient’s anatomy in real time, the patient would have likely developed an outlet obstruction.
 Figure 4
Figure 4- A 74-year-old male with neurogenic bladder underwent suprapubic catheterization. (a) AP fluoroscopic image demonstrates contrast limited to a small area (white bracket) around the catheter balloon (white arrow) on final imaging. (b) Lateral fluoroscopy image demonstrates contrast limited to the posterior pelvis, without expected extravasation inferiorly into the retropubic area. (c) Prior sagittal pelvic computed tomographic (CT) image confirming the presence of a posterior bladder diverticulum (red outline). (d) Lateral fluoroscopy image following partial deflation and retraction of the Foley balloon demonstrates contrast freely layering in the bladder, revealing the neck of the diverticulum that was previously occluded by the inflated balloon (dotted line).
One large retrospective review by Cronin et al. found a 99.6% technical success rate for primary SPC insertions placed by interventional radiologists in 549 patients, although the catheters placed ranged in size only up to 14 French. These smaller catheters, however, are prone to occlusion and often require adjustment.[14]
Lee et al. described a series of 60 patients who received an SPC under imaging guidance, similar to the technique described here, however, the catheters that their team placed were only 16–20 French.[15] Similarly, Chiou et al. described a series of 56 patients where a similar percutaneous method was used to place 18F catheters.[16] 46 of the 51 catheters placed in this series, however, were ≥26 French.
In 2015, Flynn et al. described an “inside-out” approach, safely performing a transurethral suprapubic endo-cystostomy (T-SPeC) with a new medical device (T-SPeC, Swan Valley Medical Inc., Denver, CO). They did not report any major procedure-related complications; however, the T-SPeC procedure still required anesthesia and cystoscopy, a disadvantage over the image-guided technique described here. While their cases were mostly performed in conjunction with pelvic surgery, it remains to be seen if a transurethral approach would be as successful under light sedation.[17]
The present study is limited by a lack of a control group, overall small sample size, and limited follow-up. Moreover, the technique described here is similar to that described by Papanicolou et al. in 1989 with minor differences (the peel-away sheath was advanced over the transmural balloon instead of advancement with an inner dilator). The 5-year series reported here is nonetheless notable, as there was a high technical success rate (96%), and a series of large-bore catheters of this caliber has not been previously described. The original 1989 paper did not specify catheter size (only ≥18 F) among its 15 patients, but this series demonstrates that primary percutaneous image-guided insertion of large-bore SPC in the range of 26F is a safe bladder diversion alternative and does not require staged upsizing of a prior tract. The patients in this study were given large-bore catheters at the urologist’s request as anecdotally, they have had fewer complications and required fewer repeat procedures. Nonetheless, it remains to be seen whether this larger size truly provided clinical benefit over smaller catheters.
Not all patients require such a large catheter on initial insertion; however, larger catheters are sometimes preferred or even necessary for patients who are prone to chronic hematuria/ clotting and occlusion/sedimentation.[18,19] In these patients, the interventional radiologist may be an ideal proceduralist for this task given the benefit of image utilization as a means of problem-solving as described above.
CONCLUSIONS
Various options are available for patients requiring chronic urinary bladder drainage. Suprapubic cystostomy offers many advantages including improved patient comfort, low risk of complication, and a lower risk of infection. Initial large-bore suprapubic catheter insertion has the advantage of precluding subsequent upsizing procedures. Safe percutaneous insertion of large-bore SPC in the range of 26F by radiologists trained in imaging-guided procedures is demonstrated here.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Urethral (indwelling or intermittent) or suprapubic routes for short-term catheterisation in hospitalised adults. Cochrane Database Syst Rev. :CD004203.
- [CrossRef] [PubMed] [Google Scholar]
- A comparison of outcomes of transurethral versus suprapubic catheterization after burch cystourethropexy. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:60-2.
- [CrossRef] [PubMed] [Google Scholar]
- The surgical risk of suprapubic catheter insertion and long-term sequelae. Ann R Coll Surg Engl. 2006;88:210-3.
- [CrossRef] [PubMed] [Google Scholar]
- Suprapubic cystostomy: Risk analysis of possible bowel interposition through the percutaneous tract by computed tomography. Korean J Urol. 2010;51:709-12.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound-guided suprapubic cystostomy catheter placement in the emergency department. J Emerg Med. 2004;26:319-21.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous, large-bore, suprapubic cystostomy: Technique and results. AJR Am J Roentgenol. 1989;152:303-6.
- [CrossRef] [PubMed] [Google Scholar]
- Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14:S199-202.
- [CrossRef] [Google Scholar]
- Acute urinary retention. Comparison of suprapubic and urethral catheterisation. Br J Urol. 1992;70:149-51.
- [CrossRef] [PubMed] [Google Scholar]
- Not to knot a catheter. Case report of the knotting of a suprapubic catheter. Scientific World Journal. 2007;7:1004-6.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneous intravesical knotting of urethral catheter. APSP J Case Rep. 2011;2:21.
- [Google Scholar]
- An unusual complication of suprapubic catheter migration into the left ureter. Urol J. 2018;15:140-2.
- [Google Scholar]
- Incisional hernia after suprapubic catheterization. Obstet Gynecol. 1997;89:844-6.
- [CrossRef] [Google Scholar]
- British association of urological surgeons’ suprapubic catheter practice guidelines. BJU Int. 2011;107:77-85.
- [CrossRef] [PubMed] [Google Scholar]
- Imaging-guided suprapubic bladder tube insertion: Experience in the care of 549 patients. AJR Am J Roentgenol. 2011;196:182-8.
- [CrossRef] [PubMed] [Google Scholar]
- Fluoroscopically guided percutaneous suprapubic cystostomy for long-term bladder drainage: An alternative to surgical cystostomy. Radiology. 1993;188:787-9.
- [CrossRef] [PubMed] [Google Scholar]
- Placement of large suprapubic tube using peel-away introducer. J Urol. 1995;153:1179-81.
- [CrossRef] [Google Scholar]
- Prospective study of the transurethral suprapubic endo-cystostomy (T-SPEC(®)): An “inside-out” approach to suprapubic catheter insertion. Int Urol Nephrol. 2015;47:257-62.
- [CrossRef] [PubMed] [Google Scholar]
- Does size matter? Measured and modeled effects of suprapubic catheter size on urinary flow. Urology. 2017;102:266.e1-266.e5.
- [CrossRef] [PubMed] [Google Scholar]
- Current trends in the management of difficult urinary catheterizations. West J Emerg Med. 2012;13:472-8.
- [CrossRef] [PubMed] [Google Scholar]






