Translate this page into:
Endovascular treatment of massive hemorrhage from a radiation-induced iliac artery-enteric fistula: A case report and review of the literature

*Corresponding author: Mina F. G. Isaac, Department of Radiology, Sengkang General Hospital, Singapore. minafouad367@gmail.com
-
Received: ,
Accepted: ,
How to cite this article:How to cite this article: Isaac MF, Mirpuri TM, Kumar P, Ho CL. Endovascular treatment of massive hemorrhage from a radiation-induced iliac artery-enteric fistula: A case report and review of the literature. Am J Interv Radiol 2022;6:4.
Abstract
Arterioenteric fistula is a rare cause of massive gastrointestinal tract hemorrhage. It is defined as an abnormal communication between an artery and a bowel loop. Radiation-induced iliac artery-enteric fistula (IEF) is an extremely rare subtype with only a few cases reported in the literature. Here, we present a case of successful endovascular treatment of massive hemorrhage due to radiation-induced IEF.
Keywords
Aortoenteric fistula
Arterioenteric fistula
Iliac artery-enteric fistula
Interventional radiology
INTRODUCTION
An abnormal fistulous communication between the arterial tree and the gastrointestinal (GI) tract is a rare yet serious cause of GI hemorrhage. It is associated with a high mortality rate that approaches 100 % without immediate diagnosis and intervention.[1]
Various terms have been used to describe this entity depending on the site of the abnormal connection, for example, aortoesophageal, aortogastric, aortoduodenal, aortoenteric fistula (AEF), and iliac artery-enteric fistulas (IEFs). Arterioenteric fistula (ArEF) is a more comprehensive term defined as a communication between a major artery and a bowel loop.
The majority of ArEFs involve the abdominal aorta. However, IEFs are rarely reported in the literature, with pelvic radiotherapy being a contributing factor. Here, we present a case of radiation-induced ArEF with a brief literature review of similar previously reported cases.
CASE REPORT
A 68-year-old woman of Chinese ethnicity presented to the emergency department with acute massive melena, hematochezia, hematemesis, and sharp colicky abdominal pain. She had a history of carcinoma of the cervix treated with radical hysterectomy and chemoradiotherapy. In addition, she was known to have ischemic heart disease.
On admission, she was hypotensive with a blood pressure of 72/42 mmHg and tachycardic with a heart rate of 112 beats/min. The patient was intubated and fluid resuscitated according to our hospital’s massive transfusion protocol of four units of packed cells, one unit of pooled platelets, and one unit of fresh frozen plasma.
Urgent computed tomographic (CT) abdominal angiography revealed large amounts of intraluminal hyperdense blood products distributed within the small bowel loops [Figure 1]. In addition, there was active arterial contrast extravasation from the distal external iliac artery (EIA) into the distal ileal loops in the right lower abdomen through a fistulous tract [Figures 1 and 2].

- A 68-year-old woman with radiation-induced pelvic arterioenteric fistula presented with massive melena, hematochezia, and hematemesis. Contrast-enhanced CT (CECT) abdomen and pelvis, axial (a) image demonstrates a thin linear fistulous tract (arrow) between the medial wall of the right external iliac artery and the adjacent small bowel loop with contrast layering along the dependent wall of the bowel. Coronal (b) CECT image shows dilated small bowel loops with intraluminal hyperdense material and circumferential hyperdensity (arrow) in keeping with acute contrast extravasation outlining the wall of the small bowel.
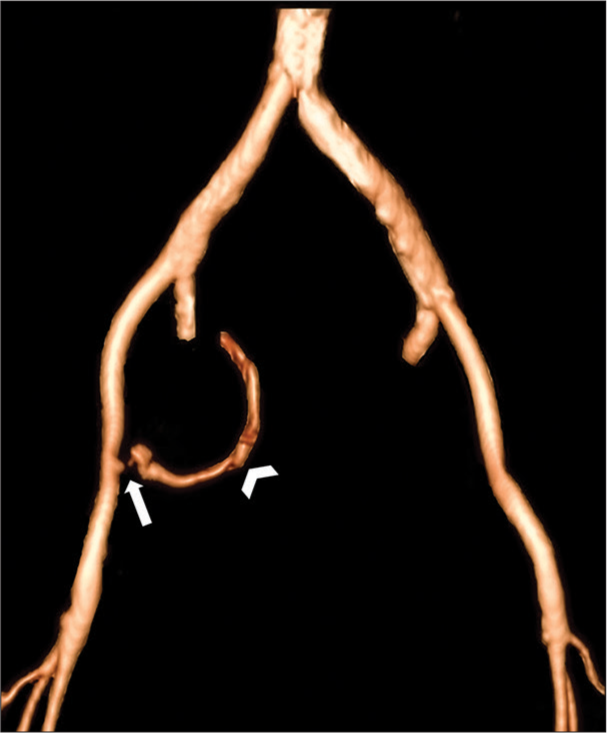
- A 68-year-old woman with radiation-induced pelvic arterioenteric fistula presented with massive melena, hematochezia, and hematemesis. Three-dimensional volume rendering techniques of the contrast-enhanced CT angiogram with bone removal demonstrate active contrast extravasation from the medial wall of the right external iliac artery (arrow) with curvilinear streak (arrowhead) corresponding to the contrast outlining the wall of an adjacent loop of the small bowel.
The patient was immediately transferred to the interventional radiology suite for urgent catheter angiography. Pelvic angiogram revealed active contrast extravasation from the medial wall of the right EIA into the small bowel [Figure 3 and Video 1] confirming the suspected fistulous communication seen on the CT angiogram. Balloon tamponade of the right EIA was rapidly performed with a 5.5 Fr Fogarty balloon catheter (Edwards, Irvine CA, USA) to provide immediate hemodynamic control. Subsequently, a 7 mm × 5 cm Viabahn covered stent (Gore®, USA) was deployed in the distal EIA successfully to exclude the fistulous tract. The size of the native external right iliac artery was 7 mm on the pre-procedure CT angiogram. A stent with a length of 5 cm was used to avoid crossing the right hip joint and make probable future surgical intervention easier. The stent did not require oversizing but to ensure a satisfactory sealing, it was post-dilated with a 7 mm angioplasty balloon. Post-procedural angiogram showed successful exclusion of the fistulous tract with no further angiographic evidence of hemorrhage [Figure 4 and Video 2].

- A 68-year-old woman with radiation-induced pelvic arterioenteric fistula presented with massive melena, hematochezia, and hematemesis. Digital subtraction angiography initial angiogram cine images confirm active contrast extravasation from a thin linear fistula at the medial aspect of the right external iliac artery (arrow).

- A 68-year-old woman with radiation-induced pelvic arterioenteric fistula presented with massive melena, hematochezia, and hematemesis. Digital subtraction angiography post-stenting angiogram cine images demonstrate cessation of contrast extravasation from the right external iliac artery.
Video 1:
Video 1:A 68-year-old woman with radiation-induced pelvic arterioenteric fistula presented with massive melena, hematochezia, and hematemesis. Digital subtraction angiography initial angiogram cine images confirm active contrast extravasation from a thin linear fistula at the medial aspect of the right external iliac artery (arrow). Video is accessible from the portal.Video 2:
Video 2:A 68-year-old woman with radiation-induced pelvic arterioenteric fistula presented with massive melena, hematochezia, and hematemesis. Digital subtraction angiography post-stenting angiogram cine images demonstrate cessation of contrast extravasation from the right external iliac artery. Video is accessible from the portal.The patient was subsequently transferred to the intensive care unit for close monitoring. She was commenced on intravenous antibiotic therapy. Post-procedure recovery was uneventful and she was discharged from the hospital several days later. There was no evidence of recurring fistulation, leak, or bleeding on her follow-up CT scans [Figure 5] up to 6 months following the procedure.

- A 68-year-old woman with radiation-induced pelvic arterioenteric fistula was treated by the endovascular stent. Follow-up contrast-enhanced CT (CECT) abdomen and pelvis at 6 weeks after stenting. Axial (a) and maximum intensity projection (b) CECT images demonstrate the right EIA stent in situ with an adjacent loop of the small bowel (arrows). There is a mild mural thickening of the adjacent small bowel loop (arrowhead), which may be related to the prior radiotherapy. No evidence of active contrast extravasation or fistulation.
DISCUSSION
ArEF is a broad term that encompasses AEF, IEF fistula, and other subcategories. ArEF can be classified into primary and secondary types.[1] The rarer primary ArEF was first described by Sir Astley Cooper in 1829. It occurs in patients who have never undergone aortic reconstruction/repair. The causes of primary ArEF include atherosclerotic aneurysms (most common), foreign bodies, tumors, trauma, radiation therapy, arteritis, and infections.[2] Secondary ArEF was first described by Brock in 1953 and occurs as a complication of aortic reconstructive surgery.[1] Being far more common than primary ArEF, the secondary ArEF can be attributed to an increasing number of abdominal aortic repairs in the past decades.[1]
The most common clinical symptoms of ArEF are abdominal pain and GI hemorrhage. GI hemorrhage may start as self-limiting intermittent episodes often termed as herald bleeds, before massive bleeding with time intervals ranging from hours to months. This can be explained by local vasospasm and hypotension with the formation of a thrombus that may temporarily occlude the fistula. Later, when the effect of local vasospasm ceases and normotension is restored, the thrombus may dislodge and lead to massive bleeding. Other less common symptoms include pulsating abdominal mass, weight loss, fever, and sepsis.
IEF following radiotherapy appears to be an extremely rare entity with the first two cases reported by Kwon et al. in 1978[3] (radiation-induced AEF was reported before that date by Zarembok, 1972). In a PubMed electronic literature search (conducted in September 2021) on “text word” synonyms for “arterioenteric fistula” and “iliac-enteric fistula” (limited to the English language), we have only found 12 case studies of radiation-induced IEF (excluded cases were describing IEF due to another etiology and AEF). These are summarized in [Table 1], including our present case.
| Author | Fistula site | Radiation site/neoplasm | Intervention | Outcome |
|---|---|---|---|---|
| Kwon et al., 1978[3] Reported two cases |
Right IIA | Cervical cancer | Ligation | COB: Yes Survival: The patient died due to respiratory failure on POD 7 |
| Left CIA to ileum | Cervical cancer | Ligation | COB: Yes Survival: Yes |
|
| Vetto et al., 1987[4] Reported three cases |
Left IIA to ileum | Rectal adenocarcinoma | Initial angioembolization Rebleeding on the 3rd day → Ligation |
COB: Yes Survival: Yes |
| Left IIA to ileal conduit and jejunum | Rectal carcinoma | Embolized with stainless steel coils Left axillofemoral bypass to restore leg perfusion |
COB: Yes Survival: Yes |
|
| Left IIA to the sigmoid colon | Carcinoma of the bladder | Ligation. | COB: Yes Survival: Post-operative course was complicated by infections, abdominal abscesses, and respiratory failure. The patient died on the POD 58 |
|
| Lukens et al., 1995[5] | Left IIA to the sigmoid colon | Cervical cancer | Not accessible | |
| Cohen et al., 2017[6] | Right internal iliac arteriovenous-enteric fistula | Rectal cancer | Embolization | COB: Yes Survival: Yes |
| Mikami et al., 2017[7] Reported two cases |
Right EIA to the small intestine | Cervical cancer | Stent | COB: Yes, rebleeding after 3 weeks → coil embolization followed by f-f bypass Survival: Yes |
| Right EIA to small bowel | Cervical cancer. | Embolization of the right iliac arteries and f-f bypass Aortic left CIA stent was placed to block blood flow into the right CIA Ligation of the distal right EIA to prevent backflow of blood through the f-f bypass |
COB: Yes Survival: Yes |
|
| Mohammad, 2018[8] | Right EIA to the small bowel | Urinary bladder carcinoma | Endovascular covered stent | COB: Yes Survival: Unknown |
| Dimech et al., 2018[2] | Right ileoduodenal fistula between the right CIA and duodenum | Ascending colon carcinoma | Endovascular covered stent | COB: Yes, massive rebleeding after 3 months Survival: The patient died shortly after rebleeding. |
| Kassamali et al., 2019[9] Indeterminate if secondary to tumor invasion or radiation-induced. |
Left CIA to adjacent small bowel | Colon and endometrial cancer | Stent | COB: Yes Survival: Yes |
| Present case | Right EIA to adjacent distal ileal loops | Cervical cancer | Endovascular covered stent | COB: Yes Survival: Yes. Referred to vascular surgery for further management of fistula |
CIA: Common iliac artery, COB: Cessation of bleeding, EIA: External iliac artery, f-f: Femorofemoral, IIA: Internal iliac artery, POD: Post-operative day
The pathophysiology of IEF is not fully understood. It may develop as a result of chronic inflammation and accelerated atherosclerosis.[4] It can also develop several years following radiotherapy.[4] The majority of cases are primary IEFs with atherosclerotic aortoiliac aneurysms and prior non-vascular surgeries being the most common causes.[4] The common iliac artery is the most frequently involved vessel.
CT angiogram is the most effective modality to detect the cause and location of GI tract bleeding. Besides active extravasation of contrast material into the bowel lumen, other typical imaging features include ectopic gas loculi adjacent to or within the involved vessel, obliteration of the fat plane between the affected vessel and bowel loop, disruption of vessel wall integrity, and focal bowel wall thickening.[1] In the majority of IEF cases, there is often evidence of contrast extravasation into the bowel lumen. In contrast for cases of AEFs, contrast extravasation into the bowel lumen is not a common feature on CT angiogram. Conventional catheter angiography is often required when the source of the hemorrhage is unclear.
Due to its rarity, there is no consensus on the most appropriate management strategy for ArEF and IEF. The goals of treatment are to control hemorrhage, prevent a recurrence, and maintain adequate distal perfusion. The management strategy has evolved over the years. Conventionally, surgical repair was considered the only treatment. However, this approach was associated with significant mortality, particularly in hemodynamically unstable patients. In recent years, endovascular repair has proved to be a safe and effective technique for rapid control of bleeding from an ArEF.[10] It can be used as a temporizing treatment that provides time for hemodynamic stabilization, control of sepsis, and acts as a bridge to definitive surgical repair.[10] Furthermore, it can be used as a long-term palliative treatment in patients who are at high risk for major surgery. Our case highlights the crucial role of endovascular repair in hemodynamically unstable patients presenting with massive hemorrhages due to an IEF. It also illustrates that endovascular repair can be used as a long-term palliative procedure.
CONCLUSION
Radiation-induced arterioenteric or IEF is a rare cause of severe lower GI bleeding which can potentially cause life-threatening complications. This condition should be suspected in patients with a prior history of pelvic radiotherapy. Prompt diagnosis facilitates early and appropriate treatment, especially when time is of the essence in an emergency setting. CT angiogram is the most effective imaging modality to diagnose GI hemorrhage and endovascular stent-graft placement is a safe and effective treatment.
Declaration of patient consent
Patient consent is not required as patients’ identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on:
References
- Aortoenteric fistulas: CT features and potential mimics. Radiographics. 2009;29:197-209.
- [CrossRef] [PubMed] [Google Scholar]
- Unusual site for primary arterio-enteric fistula resulting in massive upper gastrointestinal bleeding a case report on presentation and management. Int J Surg Case Rep. 2018;49:8-13.
- [CrossRef] [PubMed] [Google Scholar]
- Arterio-enteric fistula following pelvic radiation: A case report. Gynecol Oncol. 1978;6:474-8.
- [CrossRef] [Google Scholar]
- Iliac arterial-enteric fistulas occurring after pelvic irradiation. Surgery. 1987;101:643-7.
- [Google Scholar]
- Progressive arteriocolonic fistulization following pelvic irradiation. J Vasc Interv Radiol. 1995;6:615-8.
- [CrossRef] [Google Scholar]
- Internal iliac enteric fistula: A rare cause of hemorrhagic shock. Am Surg. 2017;83:300-1.
- [CrossRef] [Google Scholar]
- Two cases of recurrent uterine cervical cancer with arterio-enteric fistula treated by femoro-femoral artery bypass in the hybrid operation room. Int Cancer Conf J. 2017;7:26-9.
- [CrossRef] [PubMed] [Google Scholar]
- Primary right external iliac artery to small bowel fistula treated with endovascular graft. Open Access J Surg. 2018;10:555780.
- [CrossRef] [Google Scholar]
- Ileal artery pseudoaneurysm: A rare cause of the gastrointestinal bleed. J Community Hosp Intern Med Perspect. 2019;9:443-5.
- [CrossRef] [PubMed] [Google Scholar]
- Emergency endovascular “bridge” treatment for iliac-enteric fistula. Cardiovasc Intervent Radiol. 2011;34:1106-8.
- [CrossRef] [PubMed] [Google Scholar]







