Translate this page into:
Endovascular Embolectomy for Emergent Large Vessel Occlusion: A Historical Perspective

Corresponding Author: Simone Montoya, University of Rochester Medical Center, Imaging Sciences, 601 Elmwood Ave Box 648, Rochester, NY, United States. E-mail: simone_montoya@urmc.rochester.edu
-
Received: ,
Accepted: ,
How to cite this article: Montoya S, Walters E, Mai N, Bhalla T. Endovascular Embolectomy for Emergent Large Vessel Occlusion: A Historical Perspective. Am J Interv Radiol 2017, 1(2) 1-9.
Abstract
Acute ischemic stroke is one of the leading causes of morbidity and mortality in America and the leading cause of adult long-term disability. Strokes due to emergent large vessel occlusion (ELVO) often lead to significant disability; however, they also can be amenable to treatment with the potential for good functional outcome. Over a short period, the standard of treatment has evolved considerably, from supportive care to systemic therapy and now to targeted therapy. The role for mechanical thrombectomy had been debated for years, but in light of five back-to-back publications demonstrating its superiority, it is now considered standard of care in those patients who meet criteria. This article aims to introduce the reader to the progression of events leading to the current practice of endovascular embolectomy in ELVO.
Keywords
Embolectomy
thrombectomy
large vessel occlusion
stroke
stent retriever
INTRODUCTION
Astroke occurs when blood flow to the brain parenchyma is disrupted, either by rupture (hemorrhagic stroke) or by occlusion (ischemic stroke). While hemorrhagic stroke has a higher associated mortality, ischemic stroke is more common, accounting for 87% of all strokes in the United States.[1] Acute ischemic stroke remains a leading cause of morbidity and mortality worldwide, even despite advances in care. In the US, approximately 795,000 people each year experience either a new or recurrent stroke at an annual cost of more than 70 billion dollars,[1] and elderly patients fear a disabling stroke more than death.[2] The modified Rankin Score (mRS, Table 1)[3] is the tool most commonly used to assess functional outcome after stroke. Emergent large vessel occlusion (ELVO) refers to occlusion of a major intracranial artery, mainly the internal carotid artery (ICA), M1 segment of the middle cerebral artery (MCA), or basilar artery and results in the majority of cases of patients with long-term disability, generally defined in most studies as mRS ≤2.
| Score | Description |
|---|---|
| 0 | No symptoms at all |
| 1 | No significant disability despite some symptoms; able to carry out all usual duties and activities |
| 2 | Slight disability; unable to carry out all previous activities, but able to look after own affairs without assistance |
| 3 | Moderate disability; requiring some help, but able to walk without assistance |
| 4 | Moderately severe disability; unable to walk or attend to own bodily needs without assistance |
| 5 | Severe disability; bedridden, incontinent, and requiring constant nursing care and attention |
| 6 | Dead |
van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604-7
The mainstay of treatment for acute ischemic stroke is systemic intravenous (IV) thrombolysis with tissue plasminogen activator (tPA). The 1995 National Institute of Neurological Disorders and Stroke tPA stroke study demonstrated the efficacy of thrombolysis within a 3 h window in improving neurological function.[4] tPA was approved for use for in acute stroke by the Food and Drug Administration (FDA) in 1996, and since then has led to significant improvement in outcomes for patients with ischemic stroke.[5,6] Since 1997, the annual per-capita death rate from stroke in the United States has decreased by 34.3%.[1] Current American Heart Association Stroke Council (AHA/ASA) guidelines oblige administration of IV tPA to all eligible patients within 4.5 h of symptom onset, after emergency imaging to rule out hemorrhagic stroke.[7,8] Although IV tPA has been accepted for over two decades, only recently has endovascular therapy become a legitimate option. Similar to the historical trend seen with the development of percutaneous transluminal coronary angioplasty in acute coronary syndrome, earlier studies failed to demonstrate a clinical benefit of endovascular therapies over IV tPA, but in a short period several studies have firmly established the unequivocal benefit of endovascular treatment. Endovascular embolectomy for ELVO involving the anterior circulation (ICA or MCA) is now included as recommended treatment for stoke in all eligible patients by both the AHA/ASA and the Society of NeuroInterventional Surgery (SINS).[7,9]
PREHOSPITAL ASSESSMENT AND TRIAGE
Potential stroke is identified clinically based on signs and symptoms, often by emergency personnel without specialty training. The acronym FAST - which stands for face, arms, speech, and time - was initially developed in 1998 to be used by ambulance staff in the United Kingdom to identify the most common symptoms of stroke.[10] It has since been validated as a rapid assessment tool to be used outside the hospital setting and both the American Stroke Association and the National Stroke Association endorse its use by the general public. With the approval of IV tPA and exploration of endovascular treatments came the advent of organized stroke care.[11] Since 2003, The Joint Commission has certified more than 1000 hospitals as Primary Stroke Centers (PSCs) with dedicated stroke-focused programs and adherence to evidence-based clinical practice guidelines, including the capability to give IV tPA and manage the stroke patient after administration. In 2012, the Joint Commission added a higher level of certification, and there are now over 100 certified Comprehensive Stroke Centers (CSCs). In addition to all the functions of a PSC, a CSC also meets the following criteria: Availability of advanced imaging modalities, ability to care for both ischemic and hemorrhagic strokes with dedicated around-the-clock capabilities for endovascular procedures, cerebrovascular neurosurgery, and neurocritical care, evaluation of care of stroke patients using a peer review process, and participation in stroke research. At present, there is debate over whether a patient with suspected ELVO should be brought to the closest PSC, or bypass the PSC and be brought directly to a CSC.[12]
ASSESSMENT FOR ELIGIBILITY FOR TREATMENT
Once a patient with suspected stroke presents to the emergency department (ED), it must be determined whether the patient indeed is suffering from an acute ischemic stroke, or if the neurological symptoms are due to another entity. The conditions which comprise the majority (62%) of stroke mimics are postictal state, systemic infection, tumor, and toxic-metabolic disturbance.[13] The Brain Attack Surveillance in Corpus Christi Project compared the initial clinical diagnosis of stroke by ED physicians with later validation by board-certified neurologists and found the sensitivity of clinical diagnosis by emergency room physicians to be 92%, corroborating the role of the ED physician in screening for stroke and guidance for treatment decisions provided by a neurologist.[14] A meta-analysis published in 2017 including 15,721 patients again found the sensitivity and specificity of clinical diagnosis in the ED to be high (91.3% and 92.7%); however, missed diagnosis was more common in certain subgroups with milder, nonspecific, or transient symptoms on presentation.[15]
The National Institutes of Health Stroke Scale (NIHSS) is an 11-item assessment tool used to assess degree of neurologic deficit. It was originally designed as a research tool to determine baseline for clinical trials but has since been implemented in the clinical setting as a method of objective quantification of neurologic impairment. The NIHSS has been shown to predict lesion size and patient outcome and can be administered with reliable reproducibility among medical personnel. In addition, a low NIHSS score may be used to determine ineligibility for IV tPA, although the current AHA/ ASA guidelines advise against using the NIHSS as the sole criterion to withhold IV tPA in an otherwise eligible patient.[16] This is because there are certain neurologic symptoms which are not detected by the NIHSS, and a patient with a potentially devastating stroke may have a low NIHSS score.[17]
The role of imaging
The noncontrasted head computed tomography (NCCT) is the workhorse of neuroradiology, and stroke imaging is no exception. On arrival to a PSC or CSC, the patient should be directed immediately to the CT scanner to exclude the presence of intracranial hemorrhage, which would render the patient ineligible for either IV tPA or endovascular embolectomy and lead the treatment team down the path of hemorrhagic stroke. In addition, the presence of a hyperdense vessel segment or parenchymal abnormalities such as sulcal effacement, loss of gray-white differentiation, or hypoattenuation in a vascular distribution may indicate ELVO.[18]
The Alberta Stroke Programme Early CT Score systematically quantifies early ischemic changes in the MCA territory by dividing it into 10 regions.[19] The score is used on a baseline NCCT obtained before the administration of any thrombolytic. For each affected region, a point is subtracted from the best possible score (10); a score of 0 indicates diffuse MCA distribution ischemia. The ASPECT scoring system was found to be inversely related to stroke severity, and predictive for both functional outcome and symptomatic hemorrhage. In general, if more than one-third of the MCA territory (ASPECT score ≤7) is affected, thrombolysis is not offered, although the authors are quick to state that based on the available data an ASPECT score ≤7 should not in itself be the excluding criterion for thrombolysis.
Although NCCT can aid in determination of eligibility for IV tPA, often more information is needed to evaluate candidacy for endovascular intervention. Patients who benefit most from endovascular therapy have a proximal occlusive lesion and a high penumbra-to-core infarct ratio; however, the best method to determine these characteristics is unclear. In addition to NCCT, imaging modalities used to aid in the assessment of stroke include CT angiography, perfusion CT (CTP), and magnetic resonance imaging. As of yet, there is no standard set of neuroimaging that has been proven to diagnose ELVO and best predict response to endovascular treatment, and imaging protocols are determined by the availability of each modality at a given CSC. The current AHA/ASA guidelines state that noninvasive intracranial vascular imaging should be performed as soon as possible if endovascular therapy is being considered but should not delay the administration of IV tPA.[7] The specific inclusion criteria for endovascular embolectomy are listed in Table 2.
| Patients should receive endovascular therapy with a stent retriever if they meet all the following criteria Prestroke mRS score of 0-1 |
| Acute ischemic stroke receiving IV r-tPA within 4.5 h of onset according to guidelines from professional medical societies |
| Causative occlusion of the internal carotid artery or proximal MCA (M1) |
| Age >18 years |
| NIHSS score of >6 |
| ASPECTS of >6 |
| Treatment can be initiated (groin puncture) within 6 h of symptom onset |
Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al. American heart association/American stroke association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: A guideline for health-care professionals from the American Heart Association/American Stroke Association. Stroke 2015;46:3020-35
With the implementation of endovascular therapies came the need to assess posttreatment efficacy. In 2003, Higashida et al. proposed a grading system to assess angiographic response to endovascular treatment[20] based on the thrombolysis in myocardial infarction (TIMI) grading system used to assess coronary blood flow after percutaneous coronary angioplasty. The Thrombolysis in Cerebral Infarction (TICI) grading system has been inconsistently defined and applied in various trials;[21] however, it has since been modified by a consensus group in 2013 (mTICI, Table 3).[22]
| Grade | Description |
|---|---|
| 0 | No perfusion |
| 1 | Antegrade reperfusion psast the initial occlusion, but limited distal branch filling with little or slow distal reperfusion |
| 2a | Antegrade reperfusion of less than half of the occluded target artery previously ischemic territory (e.g., in 1 major division of the MCA and its territory) |
| 2b | Antegrade reperfusion of more than half of the previously occluded target artery ischemic territory (e.g., in 2 major divisions of the MCA and their territories) |
| 3 | Complete antegrade reperfusion of the previously occluded target artery ischemic territory, with absence of visualized occlusion in all distal branches |
Adapted from: Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: A consensus statement. Stroke 2013;44:2650-63
THE EVOLUTION OF ENDOVASCULAR THERAPY
Directed intraarterial (IA) thrombolysis
Enrollment began for the Prolyse in Acute Cerebral Thromboembolism (PROACT) trial in 1994, around the same time as the approval process for IV tPA was underway. PROACT showed that IA recombinant prourokinase could be delivered to recanalize occlusions of the MCA.[23] The later PROACT II trial, published in 1999, demonstrated that IA thrombolysis achieved higher rates of recanalization compared to systemic anticoagulation with heparin (66% vs. 18%; P < 0.001) and also conferred a functional benefit, measured by the mRS 90 days after injury.[24] The control arm of PROACT II would also become the historical “control” to which later, single-armed trials were compared.
The Italian synthesis expansion: A randomized controlled trial on IA versus intravenous thrombolysis in acute Ischemic stroke (synthesis expansion) trial, published in 2013, aimed to determine the superiority of IA thrombolysis compared to IV tPA with regard to survival free of disability (mRS 0-1) at 90 days.[25] Patients assigned to the endovascular treatment arm did not receive IV tPA and were treated with up to 0.9 mg/kg IA tPA into the thrombus. Although primarily a comparison between the two delivery methods (IV vs. IA) of tPA, the investigators considered this a comparison between systemic and endovascular treatment. Patients treated with IA tPA could also receive additional endovascular procedures such as mechanical disaggregation, retraction, or aspiration of the thrombus per the discretion of the intervention a list; a device was used in 56 out of 165 patients. Synthesis expansion concluded that endovascular interventions were not superior to IV tPA; 30.4% of patients in the endovascular group and 34.8% of patients in the IV tPA group were alive without disability at day 90, and each treatment group experienced symptomatic intracranial hemorrhage at a rate of 6%.
The early era of mechanical embolectomy
The era of mechanical embolectomy began with the invention of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) coil retriever (concentric medical/Stryker neurovascular, mountain view, CA, USA), also known as the MERCI device, a corkscrew-shaped, nickel titanium wire deployed at the site of the thrombus to ensnare and subsequently remove it (Figure 1). Studying the device in patients ineligible for IV tPA, investigators of the MERCI trial sought to determine whether embolectomy using the MERCI retriever could restore flow in patients presenting within 8 h of an acute ischemic stroke.[26] The TIMI score was used to assess recanalization, and treatable vessels included the intracranial vertebral artery, basilar artery, ICA, ICA terminal bifurcation, and MCA. While MERCI included more vessels than were studied in PROACT II, it demonstrated that use of the device restored vascular patency (TIMI 2-3) in 46% of patients on intention to treat analysis compared with the 18% who spontaneously recanalized in the control arm of PROACT II.[24] In 2004, the FDA approved the MERCI retriever for the “[removal of] blood clots from the brain in patients experiencing an ischemic stroke.”[27] The MERCI Retriever was billed as a complement to IV thrombolytics, which work well on small, distal clots not accessible by the device.[28] MERCI met its primary endpoint, determining that patients retreated with the retriever experienced greater rates of recanalization. Its secondary endpoint was neurological outcome, as determined by mRS at day 90. Despite higher rates of revascularization, mRS ≤2 was achieved in only 27.7% of MERCI patients, comparable to 25% of control patients in PROACT II.[24,26] For many years, contention would exist regarding whether embolectomy yielded functional benefits in patients with acute ischemic stroke over standard care despite its success in revascularization.
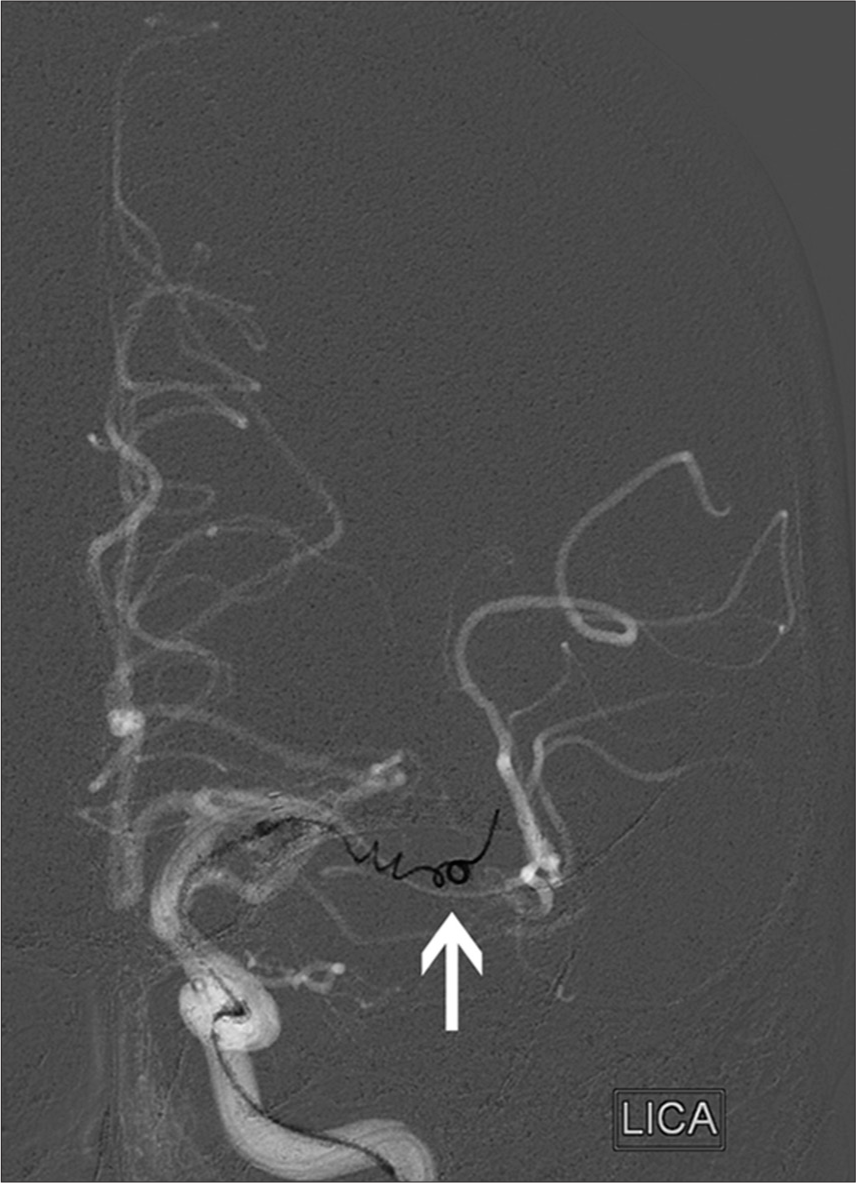
- 87-year-old woman presented with left middle cerebral artery (MCA) syndrome, National Institutes of Health Stroke Scale score of 24. Road map in AP projection from digital subtraction cerebral angiogram shows a Merci coil retriever (arrow; concentric medical/stryker neurovascular, mountain view, CA, USA) deployed within a clot in the M1 segment of the left MCA. Thrombolysis in Cerebral Ischemia grade 2b reperfusion was attained
The first randomized study attempting to prove the superiority of endovascular embolectomy began enrollment in 2004, although it was not published until 2013. Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE) sought to determine whether patients with anterior circulation strokes and a favorable “penumbral” imaging pattern would benefit from mechanical embolectomy - first using the MERCI retriever, and including newer retrieval devices later in the trial.[29] A penumbral pattern was defined as an infarct core ≤90 ml and <70% of the region at risk. Patients with a nonpenumbral pattern had a significant infarct core ≥90 ml or a small/ absent penumbra. MR RESCUE’s primary endpoint was the shift in disability levels (mRS) at day 90; secondary endpoints included good neurological outcome (mRS ≤2), revascularization determined by the thrombolysis in cerebral infarction (TICI) scale (2a-3), and reperfusion determined by CT or MR perfusion as a reduction of ≥90% in volume of the perfusion lesion. MR RESCUE found that embolectomy was not superior to standard care overall, not even in patients with a favorable penumbral pattern - regardless of treatment, patients with a penumbral pattern had lower day 90 mRS and smaller final infarct volumes.
Around the time of MR RESCUE, two other randomized and controlled trials - synthesis expansion and the third iteration of the Interventional Management of Stroke trial (IMS III) - seemed to strike down endovascular therapy as a complement or alternative to IV tPA despite improved revascularization. Synthesis expansion, described earlier, compared endovascular to systemic treatment, and determined that endovascular treatment was not superior to IV tPA on the basis of clinical benefit. IMS III sought to test the benefit of IV tPA followed by endovascular treatment compared to IV tPA alone.[30] Endovascular intervention in this trial consisted primarily by the MERCI retriever but also included IA delivery of tPA as well as other endovascular devices available at the time. The study began enrolling patients 2006 and was stopped due to futility in 2012 after finding no differences in patients’ day 90 mRS. The study did note similar safety profiles in both groups and that successful endovascular recanalization was associated with improved functional outcomes. Endovascular therapy resulted in partial to complete reperfusion in 81% of MCA occlusions (TICI 2-3) compared to previous studies reporting a 40% success rate in MCA recanalization after IV tPA.[31-33]
In the wake of multiple, negative endovascular studies, it was declared that “[t]he myth has been debunked, but the challenge is still present.”[34] Despite some technical successes in restoring vessel patency, it appeared that endovascular treatments, both IA thrombolysis and mechanical embolectomy, were not superior to standard treatment. Still, others championed the cause and blamed study design for the failure of these influential trials. Parsons and Albers believed there to be an enrollment bias in MR RESCUE with a preference for patients with larger infarcts.[35] They also cited the 2-h delay between imaging and the start of endovascular treatment, which could affect a patient’s penumbra to core ratio. The diffusion and perfusion imaging evaluation for understanding stroke evolution 2 (DEFUSE 2) study found favorable clinical outcomes in patients with a target mismatch (small diffusion-weighted imaging lesion compared to perfusion-weighted imaging lesion) in whom endovascular therapy was initiated early (median = 4.8 h from symptom onset).[36] In MR RESCUE, the mean time from last known well time to groin puncture was 6.35 h.[29] The target mismatch group in DEFUSE 2 also had an estimated core volume of 13 ml, while estimated core volumes in the penumbral pattern groups were 36 ml (embolectomy) and 37 ml (standard care). Parson and albers cited the echoplanar imaging thrombolysis evaluation trial, which suggests that good neurological outcomes were less likely to be achieved in patients with core volumes exceeding 25 ml.[37] Also of note, only 19% of embolectomy patients achieved day 90 mRS of 0-2 in MR RESCUE, compared with 44% of patients in synthesis expansion and 42% in IMS III.[25,30,38]
Promising results with stent retrievers
In IMS III, MERCI was the only retriever used for a significant part of the trial, and newer stent-like retrievers (“stentrievers”) were only utilized in a few cases before the trial was stopped.[30] Those who believed in the potential of endovascular therapy were hopeful after the advent of stentrievers, which seemed more promising than MERCI. Stentrievers apply a radial force along the length of the thrombus and incorporate clot material into stent struts on deployment (Figure 2). In 2012, two stentriever studies - the SOLITAIRE with the intention for thrombectomy (SWIFT) study and the thrombectomy RE vascularization of large vessel occlusions in acute Ischemic stroke (TREVO II) study - were published concurrently in the Lancet, which has the second-highest impact factor among medical journals. This cushioned the blow when the negative results of MR RESCUE, synthesis expansion, and IMS III were published the following year.
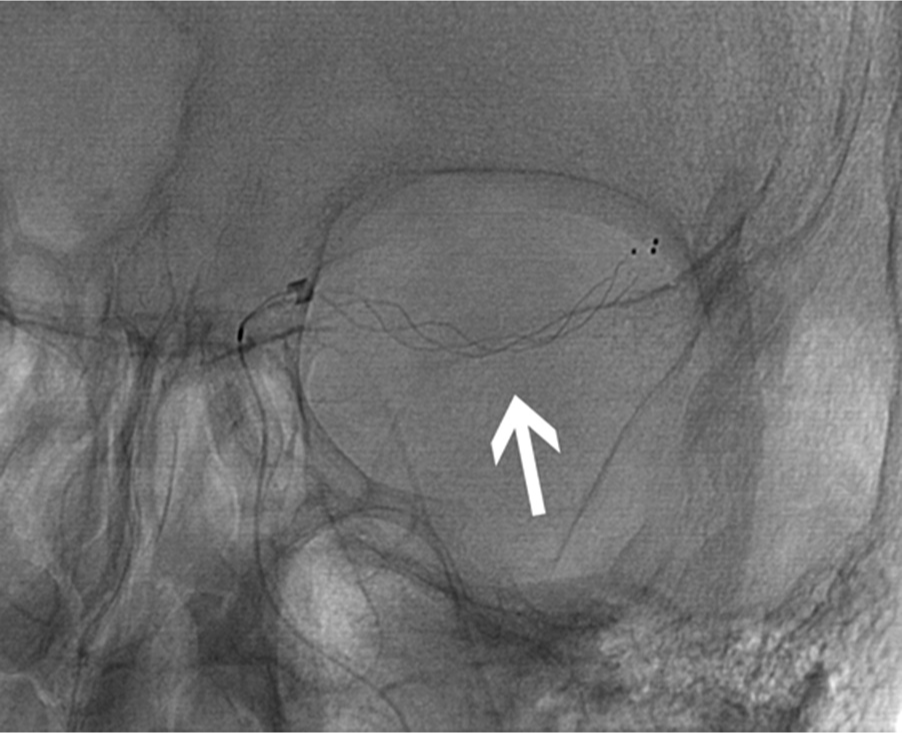
- 77-year-old woman presented with left middle cerebral artery (MCA) syndrome, National Institutes of Health Stroke Scale score of 20. Initial precontrast image in AP projection from digital subtraction cerebral angiogram shows a Trevo stent retriever (arrow; stryker neurovascular, mountain view, CA, USA) deployed within a clot in the M1 segment of the left MCA. Thrombolysis in cerebral Ischemia grade 2b reperfusion was attained
SWIFT was a noninferiority trial comparing the safety and efficacy of the Solitaire Flow Restoration Device (Covidien/ ev3, Dublin, Ireland) with the MERCI Retriever.[39] Beginning in 2010, SWIFT enrolled patients who had a persistent occlusion after treatment with IV tPA or were ineligible for IV tPA. Its primary efficacy endpoint was vascular patency (TIMI 2-3) without need for rescue therapy with another thrombectomy device and without symptomatic intracranial hemorrhage. Time to achieve recanalization and good neurological outcome at day 90 (mRS ≤2 or NIHSS improvement of ≥10 points) were secondary endpoints. SWIFT’s primary efficacy endpoint was achieved more often with solitaire (61% compared to 24% with MERCI). More patients had favorable neurological outcomes at day 90 with solitaire, and day 90 mortality was also lower in patients in the solitaire arm.
TREVO II was another randomized, noninferiority trial that began enrollment after the single-arm TREVO trial, which found that treatment with the TREVO Retriever (Stryker Neurovascular, Mountain View, CA, USA) achieved a 92% recanalization rate and that 55% of patients had good functional outcomes (mRS ≤2) at day 90,[40,41] TREVO II found that 86% of patients in the TREVO group met the primary recanalization endpoint (TICI 2-3) while 60% of patients in the MERCI group met this endpoint. Rescue therapy was required more often in the MERCI group, and vessel perforations were 10 times more common with the MERCI device, likely due to the more even force applied by the TREVO Stentriever. 40% of patients in the TREVO group had favorable outcomes at day 90 compared to 22% of patients in the MERCI group, although there were no significant differences in mortality at day 90.
2015: The year of endovascular embolectomy
SWIFT and TREVO demonstrated the superiority of stentrievers over the original MERCI coil retriever, but the superiority of endovascular therapy over IV tPA had not yet been determined. The first trial to tackle this feat since MR RESCUE was the multicenter randomized clinical trial of endovascular treatment for acute ischemic stroke in the Netherlands (MR CLEAN).[42] MR CLEAN enrolled patients between 2010 and 2014 and assessed whether endovascular treatment plus standard care would improve functional outcomes compared to standard care alone. Mechanical embolectomy included thrombus retraction, aspiration, wire disruption, or use of a retrievable stent. The study’s primary endpoint was functional outcome, assessed by mRS shift at day 90. MR CLEAN found that 32.6% of patients undergoing IA treatment were functionally independent (mRS ≤2) compared to 19.1% of patients who received standard care. All clinical and imaging secondary outcomes favored IA intervention including NIHSS at day 5-7, the absence of residual thrombus at the site of occlusion, final infarct volume, and good perfusion (TICI 2b-3). In this study, stentrievers were used in 81.5% of patients, and additional IA thrombolytics were given to 10.3% of patients.
Next came the endovascular treatment for small core and anterior circulation proximal occlusion with emphasis on minimizing CT to recanalization times (ESCAPE) trial and the extending the time for thrombolysis in emergency neurological deficits with IA therapy (EXTEND IA) trial, both published in the same issue. ESCAPE included patients with ELVO presenting within 12 h of symptom onset with NIHSS score >5 and ASPECT score >5.[43] Patients were randomized to either embolectomy with any available devices (stentrievers were recommended) along with guideline-based therapy or guideline-based therapy alone, with emphasis on rapid imaging-to-treatment times; median time from imaging to groin puncture was 51 min. The addition of embolectomy improved outcomes with an odds ratio of 1.7; 53% of patients in the intervention arm achieved a 90-day mRS ≤2, as opposed to 29% of patients who received guideline-based therapy alone. EXTEND IA included patients with anterior circulation occlusion who had received IV tPA and had a favorable imaging profile based on advanced CT or MR imaging;[44] of the 70 patients enrolled, half also underwent embolectomy with the Solitaire stentriever. Of those who received embolectomy, 71% achieved a mRS ≤2 and none experienced symptomatic hemorrhage, as opposed to 40% and 6%, respectively, who received only IV tPA.
Two more studies were published shortly thereafter - the solitaire FR with the intention for thrombectomy as primary endovascular treatment for acute Ischemic stroke (SWIFT PRIME) trial and the randomized trial of revascularization with solitaire FR device versus best medical therapy in the treatment of acute stroke due to anterior circulation large vessel occlusion presenting within 8 h of symptom onset (REVASCAT) trial - with similar results. SWIFT PRIME compared IV tPA with embolectomy using the solitaire device compared to IV tPA alone.[45] The study’s primary endpoint was mRS at day 90, which was 2 in patients receiving IV tPA and embolectomy and 3 in patients receiving IV tPA alone. 60% of patients treated with solitaire achieved functional independence (mRS ≤2) at day 90, compared to 35% of patients who received IV tPA alone. REVASCAT randomized patients presenting within 8 h of symptom onset to either best medical therapy or embolectomy in addition to best medical therapy.[46] Regarding the primary outcome, 43.7% of patients who received embolectomy had a mRS ≤2 as opposed to 28.2% of patients who did not receive embolectomy; the secondary outcome of vessel revascularization favored embolectomy. In addition, there was no difference between the two groups in terms of symptomatic intracranial hemorrhage or other significant adverse events.
The highly effective reperfusion evaluated in multiple endovascular stroke trials (HERMES) collaboration undertook a metaanalysis of all phase three trials involving stent retrievers or other second-generation embolectomy devices.[47,48] The goal was to pool the data of all the recent trials to address two questions: (1) The degree of benefit of performing mechanical embolectomy within the established 6-h window and (2) whether mechanical embolectomy past six ours was beneficial. The HERMES collaborators concluded that earlier endovascular embolectomy along with medical therapy was associated with better functional outcome at 90 days and that this became nonbeneficial after 7.3 h as compared to standard medical therapy alone.
CONCLUSIONS
The treatment of acute ischemic stroke, and specifically ELVO, has evolved dramatically in a short period. 2015 marked a turning point in regards to treatment of acute ischemic stroke due to ELVO. In light of the MR CLEAN results, ESCAPE, EXTEND IA, SWIFT PRIME, and REVASCAT were all stopped early due to an exceedance of the boundary for efficacy;[43-46] all five studies demonstrated benefit with endovascular embolectomy and were published in quick succession. Moreover, rather than being published in trade-specific journals, all of these trials were featured in the New England Journal of Medicine, which has the highest impact factor of all medical journals and is read by practitioners in all fields. AHA/ASA and SINS responded by releasing updated guidelines regarding the implementation of endovascular therapy for ELVO,[7,9] even before all the trials had been published.
Current guidelines advocate for endovascular intervention within 6 h of symptom onset, but a significant number of stroke patients present outside of this time window, either because they did not present immediately to a stroke center, or because the exact time on symptom onset is unknown. Those who fall into the latter category include those found with stroke symptoms (“unwitnessed” stroke) and those who awake from sleep with stroke symptoms (“wake-up” stroke). Results of the diffusion weighted imaging or CTP assessment with clinical mismatch in the triage of wake up and late presenting strokes undergoing neurointervention (DAWN) trial were presented at the European Stroke Organisation Conference, although they have yet to be published.[49] In this study, patients who presented between 6 and 24 h and were found to have a mismatch between clinical and imaging findings (significant neurologic deficit but small infarct size) were randomized to either embolectomy with the TREVO Retriever along with medical therapy or medical therapy alone. Preliminary findings show that 49% of patients treated with embolectomy are functionally independent at 90 days, as opposed to 13% of patients who received only medical therapy; the number needed to treat is 2.8. The DAWN study only enrolled 206 patients, however, and more evidence from additional studies will be needed before the current recommended time window can be extended. One such ongoing study is the DEFUSE 3 trial;[50] in contrast to the DAWN trial, patients enrolled in DEFUSE 3 have presented between 6 and 16 h after symptom onset, and multiple endovascular techniques and devices are included in the intervention arm.
Embolectomy is quickly becoming the preferred treatment in eligible patients. Further investigations are now directed at improving first-pass techniques to reduce time to revascularization and reduce morbidity associated with iatrogenic embolization to distal or uninvolved regions, determining the role of advanced imaging in patient selection, determining the benefit of mechanical embolectomy in those who do not meet current criteria or either systemic or endovascular therapy, and determining whether concurrent endovascular treatment of carotid stenosis is beneficial.
Declaration of patient consent
Patient's consent not obtained as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Heart disease and stroke statistics-2017 update: A report from the American heart association. Circulation. 2017;135:e146-603.
- [CrossRef] [Google Scholar]
- Patient preferences for stroke outcomes. Stroke. 1994;25:1721-5.
- [CrossRef] [PubMed] [Google Scholar]
- Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604-7.
- [CrossRef] [PubMed] [Google Scholar]
- Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581-7.
- [CrossRef] [PubMed] [Google Scholar]
- Stroke prevention and treatment. J Am Coll Cardiol. 2010;56:683-91.
- [CrossRef] [PubMed] [Google Scholar]
- Early stroke treatment associated with better outcome: The NINDS rt-PA stroke study. Neurology. 2000;55:1649-55.
- [CrossRef] [PubMed] [Google Scholar]
- American heart association/American stroke association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: A guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2015;46:3020-35.
- [CrossRef] [PubMed] [Google Scholar]
- Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke: A statement for healthcare professionals from the American heart association/american stroke association. Stroke. 2016;47:581-641.
- [CrossRef] [PubMed] [Google Scholar]
- Embolectomy for stroke with emergent large vessel occlusion (ELVO): Report of the standards and guidelines committee of the society of neurointerventional surgery. J Neurointerv Surg. 2015;7:316-21.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy of stroke referrals from primary care, emergency room physicians, and ambulance staff using the face arm speech test. Stroke. 2003;34:71-6.
- [CrossRef] [PubMed] [Google Scholar]
- Primary and comprehensive stroke centers: History, value and certification criteria. J Stroke. 2013;15:78-89.
- [CrossRef] [PubMed] [Google Scholar]
- Suspected large vessel occlusion: Should emergency medical services transport to the nearest primary stroke center or bypass to a comprehensive stroke center with endovascular capabilities? Stroke. 2016;47:1965-7.
- [CrossRef] [PubMed] [Google Scholar]
- Conditions that mimic stroke in the emergency department. Implications for acute stroke trials. Arch Neurol. 1995;52:1119-22.
- [CrossRef] [PubMed] [Google Scholar]
- A population-based study of acute stroke and TIA diagnosis. Neurology. 2004;62:895-900.
- [CrossRef] [PubMed] [Google Scholar]
- ED misdiagnosis of cerebrovascular events in the era of modern neuroimaging: A meta-analysis. Neurology. 2017;88:1468-77.
- [CrossRef] [PubMed] [Google Scholar]
- Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American heart association/ American stroke association. Stroke. 2013;44:870-947.
- [CrossRef] [PubMed] [Google Scholar]
- Is the association of national institutes of health stroke scale scores and acute magnetic resonance imaging stroke volume equal for patients with right- and left-hemisphere ischemic stroke? Stroke. 2002;33:954-8.
- [CrossRef] [PubMed] [Google Scholar]
- Early CT signs in acute middle cerebral artery infarction: Predictive value for subsequent infarct locations and outcome. Neurology. 1996;47:366-75.
- [CrossRef] [PubMed] [Google Scholar]
- Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS study group. Alberta stroke programme early CT score. Lancet. 2000;355:1670-4.
- [CrossRef] [Google Scholar]
- Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109-37.
- [CrossRef] [Google Scholar]
- What is meant by ‘TICI’? AJNR Am J Neuroradiol. 2013;34:1792-7.
- [CrossRef] [PubMed] [Google Scholar]
- Recommendations on angiographic revascularization grading standards for acute ischemic stroke: A consensus statement. Stroke. 2013;44:2650-63.
- [CrossRef] [PubMed] [Google Scholar]
- PROACT: A phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. PROACT Investigators. Prolyse in acute cerebral thromboembolism. Stroke. 1998;29:4-11.
- [CrossRef] [PubMed] [Google Scholar]
- Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: A randomized controlled trial. Prolyse in acute cerebral thromboembolism. JAMA. 1999;282:2003-11.
- [CrossRef] [PubMed] [Google Scholar]
- Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368:904-13.
- [CrossRef] [PubMed] [Google Scholar]
- Safety and efficacy of mechanical embolectomy in acute ischemic stroke: Results of the MERCI trial. Stroke. 2005;36:1432-8.
- [CrossRef] [PubMed] [Google Scholar]
- Approval of the MERCI clot retriever: A critical view. Stroke. 2005;36:400-3.
- [CrossRef] [PubMed] [Google Scholar]
- A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368:914-23.
- [CrossRef] [PubMed] [Google Scholar]
- Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893-903.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound-enhanced thrombolysis for acute ischemic stroke: Phase I. Findings of the CLOTBUST trial. J Neuroimaging. 2004;14:113-7.
- [CrossRef] [PubMed] [Google Scholar]
- Patients with acute stroke treated with intravenous tPA 3-6 hours after stroke onset: Correlations between MR angiography findings and perfusion- and diffusion-weighted imaging in the DEFUSE study. Radiology. 2008;249:614-23.
- [CrossRef] [PubMed] [Google Scholar]
- Equipoise among recanalization strategies. Neurology. 2010;74:1069-76.
- [CrossRef] [PubMed] [Google Scholar]
- A myth debunked: The results of synthesis expansion and IMS III. Neurointervention. 2013;8:1-2.
- [CrossRef] [PubMed] [Google Scholar]
- MR RESCUE: Is the glass half-full or half-empty? Stroke. 2013;44:2055-7.
- [CrossRef] [PubMed] [Google Scholar]
- MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): A prospective cohort study. Lancet Neurol. 2012;11:860-7.
- [CrossRef] [Google Scholar]
- EPITHET: Positive result after reanalysis using baseline diffusion-weighted imaging/perfusion-weighted imaging co-registration. Stroke. 2011;42:59-64.
- [CrossRef] [PubMed] [Google Scholar]
- Beyond mismatch: Evolving paradigms in imaging the ischemic penumbra with multimodal magnetic resonance imaging. Stroke. 2003;34:2729-35.
- [CrossRef] [PubMed] [Google Scholar]
- Solitaire flow restoration device versus the merci retriever in patients with acute ischaemic stroke (SWIFT): A randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241-9.
- [CrossRef] [Google Scholar]
- Neurothrombectomy for the treatment of acute ischemic stroke: Results from the TREVO study. Cerebrovasc Dis. 2013;36:218-25.
- [CrossRef] [PubMed] [Google Scholar]
- Trevo versus merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): A randomised trial. Lancet. 2012;380:1231-40.
- [CrossRef] [Google Scholar]
- A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11-20.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019-30.
- [CrossRef] [PubMed] [Google Scholar]
- Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009-18.
- [CrossRef] [PubMed] [Google Scholar]
- Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285-95.
- [CrossRef] [PubMed] [Google Scholar]
- Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296-306.
- [CrossRef] [PubMed] [Google Scholar]
- Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: A meta-analysis. JAMA. 2016;316:1279-88.
- [CrossRef] [PubMed] [Google Scholar]
- Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723-31.
- [CrossRef] [Google Scholar]
- DAWN in Full Daylight: DWI or CTP Assessment with Clinical Mismatch in the Triage of Wake-Up and Late Presenting Strokes Undergoing Neurointervention with Trevo. In: Presented at: 3rd European Stroke Organisation Conference (ESOC 2017). Prague, Czech Republic, May, 16.
- [CrossRef] [Google Scholar]
- Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3 (DEFUSE 3) NIH Stroke Net. Available from: https://www.nihstrokenet.org/clinical-trials/acute-interventional-trials/defuse-3
- [Google Scholar]






