Translate this page into:
Comparison of uterine artery embolization and myomectomy: A long-term analysis of 863 patients

*Corresponding author: Jemianne Bautista Jia, Department of Interventional Radiology, Kaiser Permanente Los Angeles, Los Angeles, California, United States. jemianne.bautista@kp.org
-
Received: ,
Accepted: ,
How to cite this article: Jia JB, Nguyen ET, Ravilla A, Mastrolonardo E, Min J, Yao JF, et al. Comparison of uterine artery embolization and myomectomy: A long-term analysis of 863 patients. Am J Interv Radiol 2021;5:1.
Abstract
Objectives:
The objective of this study is to compare the rates of secondary intervention following uterine artery embolization (UAE) versus myomectomy for the treatment of symptomatic uterine fibroids.
Material and Methods:
This is a multicenter retrospective cohort study. Eight hundred and sixty-three patients are included in this analysis, 451 patients who underwent UAE and 412 patients who underwent myomectomy between January 1, 2008, and December 31, 2012. The UAE group was significantly older than the myomectomy group with a median age of 46 versus 38 (P < 0.0001). Patient data were collected from electronic medical records between the time of their initial procedure and December 31, 2017. The primary endpoint was secondary intervention rate. Secondary endpoints included time to secondary intervention, post-procedural complications, differences in mean hemoglobin levels following the procedures, symptomatic improvement, and subsequent pregnancy outcomes. All statistical analyses were two sided and performed using SAS EG 7.13 (Cary, NC).
Results:
The median follow-up for the UAE and myomectomy groups was 7 and 7.3 years, respectively. Overall, the rates of secondary intervention were not statistically significant between the UAE and myomectomy groups, 8.9% and 11.2%, respectively (P = 0.26). However, stratified analysis in women aged 30–39 years old demonstrated an increased rate of secondary intervention in the UAE arm with a hazards ratio of 3.76 (P = 0.0099). In patients ≥40 years old, there was no significant difference in secondary intervention rate. Both groups demonstrated a significant increase in mean hemoglobin at 1 year following initial procedure with a mean difference (SD) of 1.8 (2.1) in the UAE group and 1.8 (2.5) in the myomectomy group (P < 0.0001 for both groups). The myomectomy group had a higher rate of post-procedural blood transfusion than the UAE group, 2.9% versus 0.9%, respectively (P = 0.028). Both groups had comparable rates of post-procedural pelvic infection and rehospitalization. Patients with pre-procedural menorrhagia who received UAE reported a higher rate of symptomatic improvement when compared to the myomectomy group, 75.4% versus 49.5% (P < 0.0001). Both groups reported similar rates of improvement in bulk symptoms, 46.1% and 43.2%, respectively (P = 1.0).
Conclusion:
Overall, UAE and myomectomy have comparable rates of secondary intervention during a median 7-year follow-up period. However, in women between 30 and 39 years of age, UAE resulted in higher rates of secondary intervention. UAE may be more effective in controlling patients’ menorrhagia and has lower rates of post-procedural blood transfusions.
Keywords
Fibroid
Myomectomy
Uterine artery embolization
INTRODUCTION
Leiomyomata or uterine fibroids are the most common tumor affecting women with a prevalence of more than 80% in African-American women and nearly 70% in Caucasian women by age 50.[1] Management and treatment of uterine fibroids result in an annual economic burden of up to 34.4 billion dollars in the United States.[2]
Historically, fibroids have solely been treated by hysterectomy with fibroids remaining the most common indication for hysterectomy in the United States today.[3,4] However, hysterectomy is associated with 3% rate of severe operative complications and a mortality rate of 3.8/1000 women with a higher postoperative risk in patients treated for fibroids compared to those treated for other indications.[5]
Alternative and less morbid treatments for fibroids were later developed including myomectomy.[6] Myomectomy has advantages over hysterectomy including decreased intraoperative visceral injuries and febrile morbidity. However, it has similar rates of blood loss and blood transfusion.[7] In addition, there is a high rate of recurrence of fibroids following myomectomy with studies reporting rates ranging from 21 to 51%.[8,9]
Uterine artery embolization (UAE) was first described in 1995 by Ravina et al. as an alternative therapy to surgery for patients with symptomatic fibroids and high operative risk, however later in the study, healthy women were included as well. Eleven of 16 patients reported resolution in their symptoms following the procedure.[10] A few randomized control trials, including the REST and EMMY trials, have since been performed supporting the use of UAE for the treatment of fibroids.[11,12] Most recently, Manyonda et al. published their randomized control trial comparing UAE and myomectomy in the New England Journal of Medicine. This study included 254 patients and reported similar rates of fibroid-related quality of life at 2 years and no difference in post-procedural menstrual bleeding scores between the two groups.[13]
Despite UAEs proven efficacy and benefits, it continues to be underselected as a treatment for patients with symptomatic leiomyomata. A study performed by Cardozo et al. published in 2012 found that approximately 200,000 hysterectomies and 30,000 myomectomies are performed annually for the treatment of fibroids in the United States. By comparison, just thousands of UAEs are performed in this time frame.[2] Similarly, Bonine et al. looking at treatment patterns in women in the United States with symptomatic fibroids between 2009 and 2014 found that the most common procedure performed was abdominal hysterectomy.[14]
Given this continued low proportion of patients receiving UAE in lieu of the more invasive surgical alternatives, the aim of this study was to contribute to the available objective evidence to help better guide physicians in their clinical decision-making. The purpose of this study was to compare UAE and myomectomy for the treatment of symptomatic fibroids with the primary endpoint of reintervention rate to better evaluate the durability of both procedures. A number of secondary endpoints were also evaluated including post-procedural complications, differences in mean hemoglobin levels following the procedures, symptomatic improvement, and subsequent pregnancy outcomes.
MATERIAL AND METHODS
Study design
This was an Institutional Review Board approved multicenter retrospective cohort study. Data from 13 hospitals were included in this study. Patients who received UAE or myomectomy between January 1, 2008, and December 31, 2012, were included in this study. Patients were identified using American Medical Association’s Current Procedural Terminology (CPT) codes. Patients who received UAE were identified using the CPT code 37,210. Patients who received myomectomy were identified using the following codes: 58140, 58145, 58146, 58545, and 58546. Patients were followed until they were lost to follow-up or December 31, 2017, whichever came first. To capture the complete outcomes, 5 years of continuous health plan membership were required. Gaps in enrollment of less than 90 consecutive days after the initial procedure date were allowed. Additional exclusion criteria are listed below:
Prior UAE or myomectomy
UAE and myomectomy performed same day
UAE for planned hysterectomy
UAE for postpartum hemorrhage
Myomectomy for actively prolapsing myoma
Myomectomy performed during cesarean section.
Charts were manually reviewed for application of exclusion criteria not easily identified using coding.
Initial identification of patients who received UAE or myomectomy during the study period yielded 5007 patients. Initial exclusion criteria were then applied including <5 years continuous health plan membership, prior procedures, and UAE and myomectomy performed the same day which yielded 1777 patients – 475 patients in the UAE group and 1302 in the myomectomy group. At this point, 475 patients were randomly selected from the myomectomy group as power analysis required only 426 patients in each group for determination of a statistically significant difference and to make the data extraction process more feasible. Each patient chart was then reviewed and additional exclusion criteria that were unable to be easily identified using CPT codes were applied. Ultimately 451 patients were included in the UAE group and 412 patients in the myomectomy group.
Baseline characteristics including age and race were extracted from the patient charts. Ultrasound and magnetic resonance examinations performed before initial procedures were extracted from the medical records using CPT codes and manually reviewed for number of fibroids, solitary versus multiple, and for the diameters of each patient’s largest fibroid.
Evaluation of clinical outcomes
The primary endpoint of this study was secondary intervention rate, which included hysterectomy, myomectomy, and UAE. This information was also extracted utilizing their respective CPT codes. Hysterectomy CPT codes used for the extraction algorithm are as follows: 58570, 58571, 58572, and 58573. These codes encompass open, vaginal, and laparoscopic hysterectomy procedures.
Secondary endpoints included time to secondary intervention; post-procedural complications; hemoglobin/ hematocrit before and at 3, 6, and 12 months following the procedure; improvement in menorrhagia and bulk symptoms; and pregnancy outcomes. Post-procedural complications were designated as blood transfusions, pelvic infections, and rehospitalizations occurring in the first 6 months following the procedure. Intraoperative blood transfusions were not included in post-procedural transfusions. Hemoglobin measurements, post-procedural blood transfusions, post-procedural pelvic infections, post-procedural rehospitalizations, and subsequent pregnancies were extracted using CPT codes. The presence of and improvement in menorrhagia and bulk symptoms was derived from review of history and physical and progress notes prior to the procedures and during follow-up appointments. Only patients with reported menorrhagia and/or bulk symptoms before their procedures were included in their respective analyses. Pregnancy outcomes were also derived using manual chart review by the authors.
Statistical analysis
Power analysis was performed based on previously reported rates of secondary interventions. Using 14% rate of secondary intervention for myomectomy and 8% for UAE based on prior studies, a two group χ2 test with a 0.05 two-sided significance level was calculated to have 80% power to detect the difference when the sample size in each group is 426.
For the bivariate analysis, Chi-square/Fisher’s exact tests and t-test/Wilcoxon rank-sums were used for categorical and continuous data, respectively. Time to secondary procedures following initial intervention was analyzed using Kaplan– Meier estimates. Multivariate Cox proportional hazard model was applied to detect the independent factors associated with the time to secondary procedures. Variables included in this analysis are as follows: Treatment procedure groups, age groups (30–39, 40–44, 45–49, and 50+), interaction of age group and treatment groups, adenomyosis, fibroid number, pre-procedure hemoglobin, pre-procedure hematocrit, race, blood transfusion, pre-procedure menorrhagia, and pre-procedure bulk symptoms. The stratified analysis by age groups (≤39, 40–44, and 45–49) for Cox proportional hazard model then was followed to detect the significant factors associated with the time to secondary procedures within the age groups. Since there were no patients under 30 years old for the UAE group and few patients above 49 years old in both groups, the age groups were stratified between 30 and 49 years old. A subgroup analysis was performed in patients with pre-procedure anemia (hemoglobin <12 g/dl) and in patients with and without comorbid adenomyosis. All analyses were two sided and performed using SAS EG 7.13 (Cary, NC). P < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics are detailed in Table 1. The UAE group was significantly older than the myomectomy group, with a median age of 46 (interquartile range [IQR] 42.0, 48.0) and 38 years old (IQR 34.0, 42.0), respectively (P < 0.0001). The UAE group also had a significantly higher rate of women with multiple fibroids, comorbid adenomyosis, pre-procedure menorrhagia, and patients with both pre-procedure menorrhagia and bulk symptoms. The median largest fibroid diameter was not statistically significant between the two groups, 6.2 cm in the UAE group and 7.2 cm in the myomectomy group (P = 0.56). Table 2 includes the breakdown of myomectomy procedures by type.
| UAE | Myomectomy | P-value | |
|---|---|---|---|
| n | 451 | 412 | |
| Age* | 46 (42.0, 48.0) | 38 (34.0, 42.0) | <0.00011 |
| Race, n (%) | |||
| African-American | 215 (46.7) | 155 (37.6) | 0.00132 |
| Asian/Pacific Islander | 40 (8.9) | 40 (9.7) | |
| Hispanic | 99 (22.0) | 141 (34.2) | |
| White | 90 (20.0) | 71 (17.2) | |
| Other | 7 (1.6) | 5 (1.2) | |
| Multiple fibroids, n (%) | 201 (85.5) | 109 (71.7) | <0.00092 |
| Largest fibroid diameter*, cm | 6.2 (4.7, 8.9) | 7.2 (10.0) | 0.0561 |
| Comorbid adenomyosis, n %) | 27 (6.0) | 8 (1.9) | <0.00262 |
| Pre-procedure hemoglobin*, g/dl | 11.9 (10.5, 13.0) | 12.2 (10.7, 13.0) | 0.331 |
| Patients with pre-procedural hemoglobin <12), n (%) | 238 (52.8) | 198 (48.1) | 0.491 |
| Preprocedure symptoms, n (%) | |||
| Menorrhagia | 400 (88.7) | 273 (66.3) | <0.00012 |
| Bulk symptoms | 273 (66.3) | 255 (61.9) | 0.172 |
| Both | 209 (46.3) | 150 (36.4) | 0.0312 |
| Myomectomy type | n (%) | CPT code |
|---|---|---|
| Open | 269 (65.2) | 58140, 58146 |
| Vaginal | 81 (19.7) | 58145 |
| Laparoscopic | 62 (15.0) | 58545, 58546 |
Results are summarized in Table 3. The median follow-up for the UAE and myomectomy groups was 7 and 7.3 years, respectively (P = 0.014). Both groups had comparable rates of secondary intervention, 8.9% in the UAE group and 11.2% in the myomectomy group (P = 0.26). Kaplan–Meier analysis was performed and did not show a significant difference in time to secondary intervention between the two groups [Figure 1].
| UAE | Myomectomy | P-value | |||
|---|---|---|---|---|---|
| Secondary interventions, n (%) | |||||
| Hysterectomy | 19 (4.2) | 22 (5.3) | 0.442 | ||
| UAE | 15 (3.3) | 7 (1.7) | 0.132 | ||
| Myomectomy | 6 (1.3) | 18 (4.4) | 0.00672 | ||
| Total | 40 (8.9) | 46 (11.2) | 0.262 | ||
| Complications, n (%) | |||||
| Blood transfusions | 4 (0.9) | 12 (2.9) | 0.0282 | ||
| Pelvic infection | 5 (1.1) | 1 (0.2) | 0.223 | ||
| Rehospitalization | 2 (2.7) | 12 (2.9) | 0.822 | ||
| Pregnancy outcomes, n (%) | |||||
| Pregnancies | 7 (1.6) | 97 (23.5) | <0.00013 | ||
| Cesarean | 2 (33.3) | 59 (70.2) | |||
| Ectopic | 0 (0) | 3 (3.6) | |||
| Spontaneous abortion | 1 (16.7) | 12 (14.3) | |||
| Termination | 3 (50.0) | 0 (0) | |||
| Vaginal delivery | 0 (0) | 10 (11.0) | |||
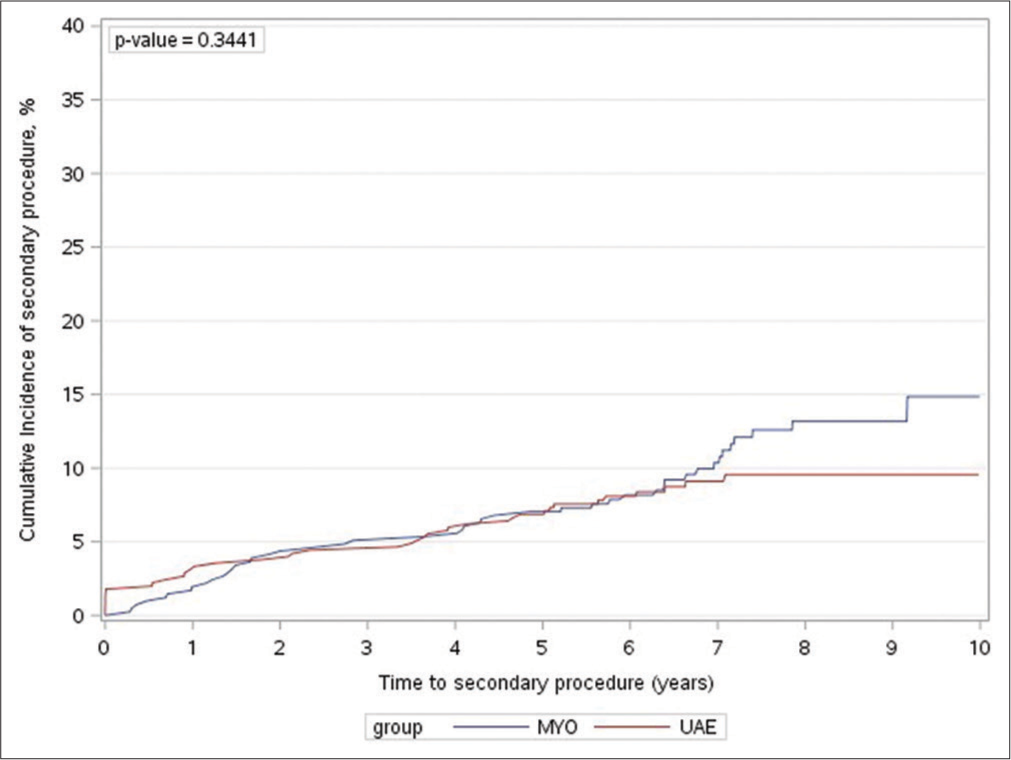
- Kaplan–Meier estimates for the cumulative incidence rate of secondary intervention. MYO: Myomectomy, UAE: Uterine artery embolization.
Multivariate Cox proportional hazard model showed the interaction of age groups and treatment groups were significant. Therefore, stratified Cox proportional hazard model by age groups was applied and the results are shown in Table 4. For age less than 40 years old group (n = 262), treatment group was a significant factor with a hazard ratio of 3.76 (P=0.0099), which indicates that the UAE group had a 3.76 times chance of undergoing a secondary procedure compared to the myomectomy group. In this age group, 14.6% of patients in the UAE group and 6.5% of patients in the myomectomy group required a secondary intervention (P = 0.080). For age between 40 and 44 years old (n = 209), age was a significant factor with hazard ratio of 0.65 (P = 0.030), which means every 1 year increase in age decreased the chance of getting secondary procedure by 35%. However, treatment group was not a significant factor and patients following UAE and myomectomy in this age group had similar rates of secondary procedures, 3.9% versus 2.0%, respectively (P = 0.47). For the 45–49 years old group (n = 219), there were are no factors detected to that were be associated with the rate of secondary procedures. Reintervention rates were similar between the two groups, 3.9% and 6.4% in the UAE and myomectomy groups, respectively (P = 0.43).
| Variable | 30–39 years old (n=239) | 40–44 years old (n=209) | 45–49 years old (n=219) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pr> Chi-sq |
Hazard ratio | 95% hazard ratio confidence | Pr> Chi-sq |
Hazard ratio | 95% hazard ratio confidence | Pr> Chi-sq |
Hazard ratio | 95% hazard ratio confidence | ||||
| Limits | Limits | Limits | ||||||||||
| Treatment group | ||||||||||||
| UAE versus MYO | 0.0099 | 3.764 | 1.375 | 10.302 | 0.9585 | 1.027 | 0.377 | 2.801 | 0.6335 | 0.783 | 0.286 | 2.141 |
| Age | 0.3015 | 0.916 | 0.777 | 1.081 | 0.0302 | 0.647 | 0.437 | 0.959 | 0.3934 | 0.875 | 0.644 | 1.189 |
| Adenomyosis | 0.9819 | 1.026 | 0.111 | 9.462 | 0.7612 | 1.389 | 0.167 | 11.58 | 0.0615 | 3.792 | 0.938 | 15.335 |
| Fibroid number | ||||||||||||
| Single versus Multi | 0.5192 | 1.519 | 0.426 | 5.414 | 0.4371 | 0.436 | 0.054 | 3.54 | 0.9765 | 0.967 | 0.107 | 8.783 |
| Pre-procedure hct | 0.8687 | 0.971 | 0.687 | 1.374 | 0.6097 | 0.881 | 0.543 | 1.431 | 0.6709 | 0.923 | 0.64 | 1.333 |
| Pre-procedure hgb | 0.7632 | 0.862 | 0.329 | 2.261 | 0.4493 | 1.687 | 0.435 | 6.545 | 0.478 | 1.417 | 0.541 | 3.711 |
| Race | ||||||||||||
| Asian/PI versus White | 0.7273 | 0.649 | 0.057 | 7.366 | 0.2892 | 0.291 | 0.03 | 2.853 | ||||
| African-American and Caucasian | 0.83 | 1.189 | 0.244 | 5.784 | 0.7128 | 0.784 | 0.215 | 2.861 | 0.9287 | 0.949 | 0.302 | 2.981 |
| Hispanic versus White | 0.486 | 1.754 | 0.361 | 8.527 | 0.6222 | 0.706 | 0.176 | 2.823 | 0.6507 | 1.31 | 0.407 | 4.218 |
| Blood transfusion | ||||||||||||
| Y | 0.1061 | 3.868 | 0.75 | 19.95 | ||||||||
| Pre-procedure menorrhagia | ||||||||||||
| Y versus N | 0.2796 | 0.556 | 0.192 | 1.612 | 0.1935 | 3.905 | 0.501 | 30.432 | 0.2446 | 0.526 | 0.178 | 1.553 |
| Pre-procedure bulk symptoms | ||||||||||||
| Y versus N | 0.4368 | 1.562 | 0.508 | 4.804 | 0.8058 | 1.147 | 0.385 | 3.412 | 0.7907 | 0.885 | 0.358 | 2.188 |
UAE: Uterine artery embolization, MYO: Myomectomy, Multi: Multiple, hct: Hematocrit, hgb: Hemoglobin, PI: Pacific Islander, Y: Yes, N: No
The myomectomy group had a 3-fold higher rate of blood transfusion during the post-operative period defined as the first 6 months following the procedure and excluding intraoperative transfusion, 2.9% versus 0.9% in the UAE group (P = 0.028). Rates of pelvic infection during the post-operative period were not statistically significant between the two groups, 1.1% in the UAE group and 0.2% in the myomectomy group (P = 0.22). Both groups also had similar rates of rehospitalization, 2.7% and 2.9%, respectively (P = 0.82).
Four hundred patients in the UAE group and 273 patients in the myomectomy group reported menorrhagia pre-procedurally. Of these patients, a significantly higher number in the UAE group reported improvement in their symptoms following the procedure, 75.4% (n = 340) versus 49.5% (n = 204) in the myomectomy group (P > 0.0001). Both groups reported similar rates of improvement in bulk symptoms, 46.1% and 43.2% (P = 1.0), respectively.
In patients with pre-procedural anemia defined as a hemoglobin <12 g/dl (n = 227 in the UAE group, n= 190 in the myomectomy group), both groups experienced a significant rise in mean hemoglobin at one year. The mean hemoglobin rose by a mean difference (SD) of 1.8 (2.1) in the UAE group and 1.8 (2.5) in the myomectomy group (P < 0.0001 for both groups). The difference between the two groups was not statistically significant.
Six patients in the UAE group became pregnant following the procedure. Of these patients, three terminated their pregnancy, two had cesarean deliveries, and one had a spontaneous abortion. Of the patients in the myomectomy group, 84 became pregnant and 81.2% (n = 69) of these patients had successful cesarean or vaginal deliveries.
In patients who had pre-procedure MRI examinations (n = 451 in the UAE group and n = 412 in the myomectomy group), patients who had comorbid adenomyosis were identified and subgroup analysis was performed. Twenty-seven patients in the UAE group and 8 patients in the myomectomy group had comorbid adenomyosis confirmed on MRI. Three patients in each of these groups underwent a secondary procedure for a rate of 11.1% in the UAE group and 37.5% in the myomectomy group (P = 0.12). The group of patients with comorbid adenomyosis who underwent UAE reported a significantly higher rate of improvement in menorrhagia, 85.3% versus 50% in the corresponding myomectomy group (P = 0.028). Both groups reported similar rates of improvement in bulk symptoms, 48.1% and 50% (P = 0.68), respectively.
DISCUSSION
This study demonstrated comparable rates of secondary interventions following UAE and myomectomy over a 7-year follow-up period. However, stratified analysis by age group demonstrated a higher rate of secondary intervention following UAE in women 30–39 years of age. Women 40–49 years of age had similar rates of secondary intervention. UAE was also shown to result in lower rates of blood transfusion in the post-operative period and may be more effective in the treatment of symptomatic menorrhagia.
The increased rate of secondary intervention following UAE in patients age 30–39 years old is something that should be discussed with patients in this age group during the treatment decision-making process and weighed with the other attributes of the two procedures. Counterintuitively, although UAE is associated with higher rates of secondary interventions in this age group, patients reported higher rates of improvement in menorrhagia and equivalent rates of improvement in bulk symptoms compared to the myomectomy group. The reasons for this phenomenon cannot be definitively elucidated on retrospective review, however, may be related to the less invasive nature of UAE resulting in increased willingness to undergo a second procedure. Women 30–39 years old in the myomectomy group may also still desire fertility making them more hesitant to undergo a second procedure. Women who receive UAE are screened to ensure they do not desire future fertility and therefore they may be more inclined to undergo repeat procedures if their symptoms persist. In patients 40 years of age and older, the two procedures are equivalent in rate of secondary procedures. The equilibration at later ages is likely attributable to closer temporal relationship to menopause which is associated with improvement in fibroid symptoms.
The rate of secondary interventions for the UAE group was lower than rates reported in the randomized EMMY and REST trials. Five-year results from the EMMY and REST trials reported secondary intervention rates of 28.5% and 32%, respectively.[11,12] This may be related to improved techniques as those trials were performed in the early 2000s shortly after UAE became an accepted treatment for fibroids. The majority of physicians performing UAEs in the EMMY trial actually performed less than 10 UAEs before the trial. In addition, improved technology may be contributory as polyvinyl alcohol (PVA) particles were the primary embolic agent used in the EMMY and REST trials. All patients in this study received tris-acryl gelatin microspheres (Embosphere, Merit Medical Systems Inc., South Jordan, Utah), which have been shown to be more effective than PVA particles in fibroid infarction.[15] During the EMMY trial, 4 French or 5 French catheters were used for embolization, whereas now and in the majority of cases in the study, microcatheters are primarily used which may also contribute to improved outcomes. More recent studies report lower reintervention rates similar to the results of this study including the study performed by Scheurig-Muenkler et al. published in 2013, which included 308 patients and reported a reintervention rate of 15% at 5 years.[16] Manyonda et al. in their recent randomized control trial reported a similar rate of reintervention in their UAE group of 15% at 2 years.[13]
The rate of secondary intervention for the myomectomy group was similar to those reported in the literature. In the REST trial, only five patients in the myomectomy group had 5-year follow-up and of these only one required a subsequent myomectomy for a rate of 20%.[11] Additional studies looking at laparoscopic myomectomy reported reintervention rates at 5 years between 6 and 14%.[9,17,18]
The rates found for improvement in menorrhagia and bulk symptoms differed from previously reported rates. However, the literature overall varies widely. Rates of improvement in menorrhagia following UAE range between 51.2% and 92%.[12,19-21] Hamoda et al. reported 51.2% rate of improvement in menorrhagia at 9–14 years following UAE in a study published in 2016 with a total of 197 patients.[19] The Emmy trial reported a rate of 76% at 2 years.[12] These rates overall have a downward trend based on the length of the follow-up period. The median follow-up of patients in this study was 7 years and symptomatic improvement was gauged based on follow-up examinations during this time. This may help to explain why the rate of improvement in menorrhagia is at the lower end of this range.
In terms of rates of improvement in bulk symptoms following UAE, the reported rates range between 48.8% at 9–14 years by Hamoda et al. and 66.2% at 2 years in the EMMY trial.[12,19] Again, a similar downward trend with time is noted and the results of this study are similar to the rates reported by Hamoda et al. at 9–14 years.
This study reported similar to slightly higher rates to those reported in the literature in terms of symptomatic improvement following myomectomy. Obed et al. reported an improvement in menorrhagia of 33% in patients following myomectomy.[22] Kramer et al. in a randomized control trial reported improvement in symptoms in 24% of patients in their myomectomy group, which included bulk symptoms and menorrhagia.[23]
The differences in rates between patients without symptomatic improvement and secondary interventions are multifactorial and based on patient preferences. Women without improvement in their symptoms who did not undergo a secondary procedure may have made that decision based on unwillingness to undergo a second invasive procedure, preference for medical therapy, and possible negative attitude toward the procedures given their lack of improvement. The improvement in menorrhagia following UAE in patients with comorbid adenomyosis was even higher than that of the overall group. The rate of improvement in menorrhagia in the patients following myomectomy was similar in patients with adenomyosis and the overall group. This suggests that UAE may be a preferable treatment for patients with fibroids and comorbid adenomyosis. The efficacy of UAE in the treatment of patients with adenomyosis has been described in the literature.[24]
Patients in the UAE group were more likely to get a second UAE or hysterectomy, whereas the patients in the myomectomy group were more likely to have a second myomectomy or hysterectomy. This is likely due to the personal preferences and practice patterns of individual clinicians involved in the patients’ care. In the future, it will be important to look at tertiary outcomes to see if these clinical practices can be changed and procedures chosen based on objective standards.
Despite the large patient cohort, only six patients in the UAE arm became pregnant during the follow-up period. This is likely secondary to the older age of this group and because patients who receive UAE are prescreened for the desire to preserve fertility. There continues to be a paucity of data regarding pregnancy outcomes in patients following UAE. Research performed by Pisco et al. looking at partial and complete UAE in patients who were unable to conceive which included 359 women actually found that UAE may improve fertility with a successful spontaneous pregnancy rate of 36.7% at 2 years.[25] Larger patient datasets still need to be collected to validate these outcomes and to evaluate whether UAE is a safe option to offer patients desiring to preserve fertility. Given UAEs benefits when compared to myomectomy, it would be a good alternative for those women if it does not compromise future pregnancies.
The main strengths of this study are the large patient cohort, 7-year follow-up period, and multicenter involvement. The largest limitation of this study is the significant age difference between the two groups, which was mitigated through stratified analyses, and the retrospective study design. The use of CPT and ICD codes to capture outcomes such as secondary intervention rate and complications may also lead to error due to cases of misclassification. This study also only involved hospitals in the South California region and all patients were insured which may limit external validity. Location of the fibroids (submucosal, intramural, or subserosal) was difficult to attain consistently from the imaging reports and was unable to be included in the analysis.
Most commonly, patients present for consultation for uterine fibroids with menorrhagia and bulk symptoms. Given the results of this study, UAE may be the natural choice for many patients. In this patient subset, UAE resulted in higher rates of reported improvement in menorrhagia on follow-up visits when compared to myomectomy and comparable rates of improvement in bulk symptoms. In addition, UAE resulted in a lower rate of blood transfusions. This data should be considered by all clinicians counseling women in the treatment of their symptomatic uterine fibroids.
CONCLUSION
Overall, UAE and myomectomy have comparable rates of secondary intervention during a median 7-year follow-up period. However, in women between 30 and 39 years of age, UAE resulted in higher rates of secondary intervention. UAE may be more effective in controlling patients’ menorrhagia and has lower rates of post-procedural blood transfusions.
Acknowledgments
The findings from this study were presented as an oral presentation at the Society of Interventional Radiology Annual Scientific Meeting in Austin, Texas, March 23–28, 2019.
Declaration of patient consent
Institutional Review Board permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- High cumulative incidence of uterine leiomyoma in black and white women: Ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-7.
- [CrossRef] [PubMed] [Google Scholar]
- The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206:211.e1-9.
- [CrossRef] [PubMed] [Google Scholar]
- Hysterectomy rates in the United States 1990-1997. Obstet Gynecol. 2002;99:229-34.
- [CrossRef] [Google Scholar]
- Hysterectomy rates for benign indications. Obstet Gynecol. 2006;107:1278-83.
- [CrossRef] [PubMed] [Google Scholar]
- Severe complications of hysterectomy: The value study. BJOG. 2004;111:688-94.
- [CrossRef] [PubMed] [Google Scholar]
- Relative morbidity of abdominal hysterectomy and myomectomy for management of uterine leiomyomas. Obstet Gynecol. 1996;88:415-9.
- [CrossRef] [Google Scholar]
- Long-term risk of fibroid recurrence after laparoscopic myomectomy. Eur J Obstet Gynecol Reprod Biol. 2014;180:35-9.
- [CrossRef] [PubMed] [Google Scholar]
- Recurrence rate after laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 1998;5:237-40.
- [CrossRef] [Google Scholar]
- Randomised comparison of uterine artery embolisation (UAE) with surgical treatment in patients with symptomatic uterine fibroids (REST trial): 5-year results. BJOG. 2011;118:936-44.
- [CrossRef] [PubMed] [Google Scholar]
- Uterine artery embolization vs hysterectomy in the treatment of symptomatic uterine fibroids: 10-year outcomes from the randomized EMMY trial. Am J Obstet Gynecol. 2016;215:745.e1-12.
- [CrossRef] [PubMed] [Google Scholar]
- Uterine-artery embolization or myomectomy for uterine fibroids. N Engl J Med. 2020;383:440-51.
- [CrossRef] [PubMed] [Google Scholar]
- Contemporary treatment utilization among women diagnosed with symptomatic uterine fibroids in the United States. BMC Womens Health. 2020;20:174.
- [CrossRef] [PubMed] [Google Scholar]
- Leiomyoma infarction after uterine artery embolization: A prospective randomized study comparing tris-acryl gelatin microspheres versus polyvinyl alcohol microspheres. J Vasc Int Radiol. 2008;19:58-65.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical long-term outcome after uterine artery embolization: sustained symptom control and improvement of quality of life. J Vasc Interv Radiol. 2013;24:765-71.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of leiomyoma recurrence after myomectomy. Obstet Gynecol. 2005;105:877-81.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of leiomyoma recurrence after laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14:690-7.
- [CrossRef] [PubMed] [Google Scholar]
- Intermediate and long-term outcomes following uterine artery fibroid embolization. Eur J Obstet Gynecol Reprod Biol. 2015;191:33-8.
- [CrossRef] [PubMed] [Google Scholar]
- Pregnancy after uterine artery embolization for leiomyomata: The Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
- [CrossRef] [PubMed] [Google Scholar]
- Initial experience with use of tris-acryl gelatin microspheres for uterine artery embolization for leiomyomata. J Vasc Interv Radiol. 2001;12:1059-63.
- [CrossRef] [Google Scholar]
- The benefit of myomectomy in women aged 40 years and above: Experience in an urban teaching hospital in Nigeria. Niger Med. 2011;52:158-62.
- [CrossRef] [PubMed] [Google Scholar]
- Interim analysis of a randomized controlled trial comparing laparoscopic radiofrequency volumetric thermal ablation of uterine fibroids with laparoscopic myomectomy. Int J Gynaecol Obstet. 2016;133:206-11.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term results of uterine artery embolization for symptomatic adenomyosis. AJR Am J Roentgenol. 2007;188:176-81.
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneous pregnancy with a live birth after conventional and partial uterine fibroid embolization. Radiology. 2017;285:302-10.
- [CrossRef] [PubMed] [Google Scholar]







