Translate this page into:
Combined transarterial embolization and percutaneous image-guided ablation for the treatment of T1B and central renal tumors in patients with high surgical risk

*Corresponding author: Francisco Donato, Department of Radiology, University of Iowa, Carver College of Medicine, Iowa City, Iowa, United States. francisco-donato@uiowa.edu
-
Received: ,
Accepted: ,
How to cite this article: Donato F, Ahrar K, Karam J, Matin S, Abdelsalam ME, Sabir S. Combined transarterial embolization and percutaneous image-guided ablation for the treatment of T1B and central renal tumors in patients with high surgical risk. Am J Interv Radiol 2022;6:11.
Abstract
Objectives:
The objectives of the study were to assess the technical success, efficacy, and complications of the combination of transarterial embolization (TAE) followed by percutaneous ablation in treating stage cT1b and central renal tumors.
Material and Methods:
An institutional registry was reviewed for patients who underwent TAE followed by percutaneous computed tomography (CT)-guided renal ablation from 2007 to 2018. Twenty-eight consecutive patients (median age 69 years; range 45–89 years) with tumor size ranging from 25 to 58 mm (average 45 mm), including 24 patients with T1b tumors and four with central T1a tumors, were identified. Nineteen patients had TAE followed by cryoablation (CA), and nine patients had TAE followed by radiofrequency ablation (RFA). Technical success, local tumor control, and post-procedure complications were retrospectively reviewed.
Results:
All procedures were technically successful. Local tumor control was achieved in 26 of 28 patients (92.9%) at a median follow-up of 26 months. Local tumor recurrence was found in two of 28 patients, with a median time to local recurrence of 15 months. The recurrences occurred in one patient who had TAE plus CA and in one patient who had TAE plus RFA. Self-limited hematoma and hematuria were minor complications observed in 3 patients (10.7%). Only one patient had a major complication. This patient developed ureteral obstruction and perirenal abscess requiring ureteral stent and percutaneous drainage without long-term sequela.
Conclusion:
In this cohort of patients, a combination of TAE and percutaneous CT-guided ablation was an effective, well-tolerated, and safe treatment for patients with T1b and central renal tumors.
Keywords
Combined ablation and embolization
Cryoablation
Locoregional treatment of renal cell carcinoma
Radiofrequency ablation
Transarterial embolization
INTRODUCTION
Annually, over 430,000 new cases of renal cell carcinoma (RCC) are diagnosed worldwide, and almost 180,000 patients die from the disease.[1] Renal cancer incidence has increased at least partially due to incidental detection of renal masses when abdominal imaging is performed for unrelated reasons.[2,3] Most people with kidney cancer are elderly, aged between 65 and 74.[1] Patients with hereditary RCC syndromes develop kidney cancer at an earlier age, and more often, the tumors are multifocal and bilateral.[4,5] Surgical removal is the gold standard for localized RCC.[6,7] However, many patients have an unacceptable risk for surgery due to old age and multiple comorbidities. Other patients may be unfit for surgical resection because of multiple tumors, bilateral tumors, or solitary kidneys and high risk for complete loss of renal function after partial nephrectomy. For those patients, percutaneous radiofrequency ablation (RFA) or cryoablation (CA) is a minimally invasive alternative for curative treatment.[7,8]
Long-term cohorts and controlled clinical trials have demonstrated comparable 5-year disease-free survival and cancer-specific survival for patients with T1a renal cancer undergoing RFA versus nephrectomy.[9-12] The American Urological Association guidelines support thermal ablation as an alternate approach for the management of cT1a renal masses <3 cm in size.[8] There is an increased likelihood of residual disease for renal tumors >3.5 cm and central tumors treated with percutaneous ablation.[13] Furthermore, larger and centrally located tumors are associated with a higher risk of complications after percutaneous ablation.[13-15] The hypervascularity of most RCCs provides a target for adjunctive therapy with transarterial embolization (TAE) before thermal ablation to decrease thermal sink, increase tumor ischemia, and decrease post-procedural bleeding. The small cohorts available in the literature suggest that combined TAE and thermal ablation can improve outcomes for patients with larger or central RCC.[16-19] The purpose of this study was to review the experience of a single quaternary health care institution and evaluate the technical success, efficacy, complications, and survival outcomes of the combination of TAE followed by percutaneous computed tomography (CT)-guided CA or RFA in treating Stage cT1b and central renal tumors.
MATERIAL AND METHODS
Institutional Board Review approval and informed consent waiver were obtained to review our institutional database in compliance with the HIPAA. All percutaneous image-guided renal TAE followed by percutaneous ablation procedures performed between March 2007 and December 2018 were reviewed. Twenty-eight patients were treated with combined TAE and percutaneous ablation (CA or RFA) for T1b or central renal tumors during this period. Patients were selected for percutaneous treatment after evaluation in conjunction with our institution’s urology department. Treated patients were considered a high surgical risk due to older age, previous renal surgery, or substantial comorbidities.
Of this cohort, 26 patients (92.3%) had biopsy-proven RCC and 2 (7.7%) had imaging consistent with RCC. Nineteen patients (67.9%) were treated with TAE followed by CA, and 9 patients (32.1%) were treated with TAE followed by RFA. The first 15 patients underwent TAE followed by next-day ablation and the last 13 underwent TAE with same-day ablation. All patients were treated in a single percutaneous ablation session.
Embolization procedures
Embolization was performed under moderate sedation for patients who underwent TAE with next-day ablation and with general anesthesia for those who underwent same-day TAE and ablation. The embolization procedures were performed by one of five interventional radiologists with between 2 and 19 years of experience in embolization. All patients underwent catheterization of the renal artery followed by superselective catheterization of segmental arteries supplying the tumors using a coaxial microcatheter. The embolization material was selected at the interventional radiologist’s discretion. A mixture of ethanol and ethiodized oil (Guerbet, Princeton, New Jersey) was used in 13 patients (46.4%); ethanol alone in 9 patients (32.1%); ethanol and 100 µm and 250 µm Embozene microspheres (Boston Scientific, Natick, Massachusetts) in 2 patients (7.1%); ethanol and absorbable gelatin sponge (Ethicon, Somerville, New Jersey) in 1 patient (3.6%); 100 and 250 µm Embozene microspheres in 2 patients (7.1%); and absorbable gelatin sponge alone in 1 patient (3.2%). The endpoint of embolization was the stasis of the feeder arteries that supplied the tumors.
Ablation procedures
All ablation procedures were performed under general anesthesia with CT guidance by one of two interventional radiologists with 6 and 19 years of experience in percutaneous ablation. Details of the technique are described somewhere else.[20]
RFA procedures
RFAs were performed using a Cool-tip RFA System with a switching controller (Medtronic, Minneapolis, Minnesota) with up to three internally cooled electrode applicators. The generator power was progressively increased with each applicator activated individually until the rapid elevation of the impedance was noted. Then, all applicators were activated simultaneously using the switching controller until rapid cycling between the applicators was noted. The generator was operated in the impedance control mode to minimize tissue desiccation and vaporization and achieve temperatures between 60°C and 90°C, with each ablation cycle lasting from 8 to 12 min. The number of overlapping ablations was based on tumor size and morphology, aiming to create a zone of ablation that extended at least with a 5 mm safety margin.
CA procedures
All CA procedures were performed using an Endocare Cryocare System (HealthTronics, Austin, Texas). Multiple Perc-17 or Perc-24 cryoprobes were placed 10–20 mm apart to produce a contiguous ice ball achieving at least 5 mm of safety margin. The median number of CA probes used was 4 (range 3–8). The cryoprobes were set up 100% when deemed safe, and, subsequently, the power on each cryoprobe was adjusted by the operator to control the size and shape of the ice ball. Each ablation included at least two freeze cycles separated by an active thaw. CT imaging was performed at least every 3–5 min interval during the freeze cycles to evaluate the extension of the ice ball. The typical duration of the freeze and thaw cycles was 10 min and 8 min, respectively.
Unless contraindicated, contrast-enhanced CT imaging, including an arterial, nephrographic, and excretory phase, was obtained at the end of the ablation to evaluate the technical success and any periprocedural complications. Hydrodisplacement of the adjacent structures, including bowel, liver, spleen, pancreas, and retroperitoneal nerves, was performed for 12 patients (42.9%). Pyeloperfusion to prevent thermal injury of the collecting system was performed in 2 patients (7.1%) using a retrograde ureteral stent placed by urology before the procedure. A core biopsy of the tumor was obtained immediately before the ablation using an 18-gauge coaxial biopsy needle in all but two patients without a previous biopsy showing RCC.
Post-procedure follow-up
All patients were admitted overnight after the ablation for monitoring. Patients were followed preferably with contrast-enhanced CT initially performed 3–6 months after the procedure, every 6 months until 2 years, and then annually.[21] Independent readers from the radiology department read the follow-up CT scans. Magnetic resonance imaging was used for follow-up in patients with contraindication to iodine intravenous contrast.
Assessment and terms
Central tumors were defined as endophytic tumors invading the renal sinus fat without an exophytic component. Exophytic tumors were those with epicenter outside the renal contour and without renal sinus invasion, and mixed tumors were all others.[22] Clinical success was defined by the absence of nodular enhancement in the ablation site at the end of the ablation and the first follow-up images. Recurrence was defined as a new local nodular-enhancing tumor identified after the initial follow-up cross-sectional images or new metastatic disease. Complications were categorized per the revised Clavien–Dindo system, and any Grade II or higher were considered a major complication.
Statistical methods
Continuous features are summarized as medians, ranges, and interquartile ranges; categorical characteristics are summarized with frequency counts and percentages. The method of Kaplan–Meier was used to estimate the distribution of overall survival and time to disease recurrence from the embolization date. Patients who lost to follow-up or died without recurrence were censored at their last follow-up or death dates. Fisher’s exact tests were used to compare the distribution of categorical parameters between groups. Statistical analyses were performed using R version 3.6.0. All statistical tests used a significance level of 5%.
RESULTS
The summary of the patient’s characteristics is listed in [Table 1]. The median length of stay was 2 days (range 1–6 days, interquartile range 2–2.2 days). The most of the patients were discharged in the morning after the ablation. Thus, the first 15 patients of the cohort stayed at least two nights. There were no deaths within 30 days after the procedure and no deaths caused by the primary renal tumors. Only four patients in this cohort had T1a tumors, and only one tumor was <3 cm. These patients were elected to receive TAE before ablation due to the challenge in treating central tumors and the higher recurrence rate of these lesions.[14] [Table 2] lists the summary of tumor characteristics.
| Age (year) | N | % |
|---|---|---|
| Median | 69 | |
| Mean±SD | 69±12 | |
| Range | 45–89 | |
| Gender | ||
| Male | 21 | 75 |
| Female | 7 | 25 |
| Solitary kidney | ||
| No | 27 | 39.3 |
| Yes | 1 | 32.1 |
| Previous renal surgery | ||
| No | 24 | 85.7 |
| Yes | 4 | 14.3 |
| Chronic kidney disease | ||
| No | 10 | 35.7 |
| Stages 1–2 | 10 | 35.7 |
| Stage 3 | 7 | 25 |
| Stages 4–5 | 1 | 3.6 |
| Other comorbidities | ||
| CHF/CAD | 12 | 42.9 |
| Diabetes | 14 | 50 |
| COPD | 6 | 21.4 |
| Morbid obesity | 12 | 42.9 |
| Other cancer (non-renal) | 6 | 21.5 |
SD: Standard deviation, CAD: Coronary artery disease, CHF: Congestive heart failure, COPD: Chronic obstructive pulmonary disease, TAE: Transarterial embolization
| Tumor size | N | % |
|---|---|---|
| Median | 4.5 cm | |
| Average | 4.5 cm | |
| Interquartile range | 4.3–4.7 cm | |
| Range | 2.5–5.8 cm | |
| Tumor stage | ||
| T1B | 24 | 85.7 |
| T1A (central) | 4 | 14.3 |
| RCC subtype | ||
| Clear cell | 23 | 82.1 |
| Papillary | 2 | 7.1 |
| Chromophobe | 1 | 3.6 |
| No biopsy | 2 | 7.1 |
| Parenchymal location | ||
| Mixed | 13 | 46.4 |
| Central | 9 | 32.1 |
| Exophytic | 6 | 21.4 |
| Location to polar line | ||
| Interpolar | 11 | 39.3 |
| Superior | 9 | 32.1 |
| Inferior | 8 | 28.6 |
| Other renal tumors | ||
| No | 24 | 85.7 |
| Yes | 4 | 14.3 |
TAE: Transarterial embolization, RCC: Renal cell carcinoma
Technical success and recurrences
Technical success with complete tumor ablation was achieved for all 28 treated tumors (100%) in one treatment session. Local tumor control was achieved in 26 of 28 patients (92.9%) at a median follow-up time of 26 months (range 1–109 months). Recurrence was detected in 2 of 28 patients (7.1 %) at a median time of 15 months (range 7–23 months). None of the patients developed distant metastasis from the primary renal tumor. The two patients with local recurrence at the reporting time were considered inappropriate for further treatment. One patient with recurrence 23 months post-TAE plus RFA died of a metastatic synovial sarcoma after 45 months of follow-up. The second patient, with recurrence 7 months post-TAE plus CA, was placed on pazopanib considering his advanced age (81 years) and concurrent lymphoma. In 16 of the 28 patients, glomerular filtration rate (GFR) was available pre-procedure and at ≥6 months of treatment. In this group, the mean GFR was 79.7 at baseline and 66.7 at ≥6 months of treatment. Nine patients had chronic kidney disease stage ≥3 at the time of the procedure. There was no significant change in chronic kidney disease stages at ≥6 months post-treatment compared with the baseline (P = 0.48). No patients required initiation of dialysis. [Figure 1] shows an example of long-term disease control.

- A 68-year-old man with clear cell renal cell carcinoma. Long-term follow-up after combined renal embolization and cryoablation. (a) Axial contrast-enhanced computed tomography (CT) image shows 4.5 cm right RCC, which invades the renal sinus (arrow). (b) A subselective angiogram of a branch of the right renal artery with a microcatheter during the embolization procedure shows the highly vascular renal mass (open arrow). (c) Axial CT image during the cryoablation shows the ice ball extending beyond the mass, a total of five cryoprobes were used. (d) Axial contrast-enhanced CT image obtained 68 months after the ablation shows the ablation site with no recurrent tumor (arrowhead).
Procedural complications
In this cohort, four patients developed complications; 3 patients (10.7%) had minor complications (Clavien–Dindo Grade I) needing only conservative management. Minor complications included self-limited perirenal hematoma; hematuria, and one patient developed perirenal hematoma and hematuria. One patient (3.6%) developed a major complication (Clavien–Dindo Grade IIIa). This patient presented with obstruction of the ureteropelvic junction from clot requiring placement of a nephroureteral catheter later converted to a ureteral stent, which was removed after 4 months. He also developed a perirenal abscess 4 weeks after treatment which required percutaneous drainage and antibiotics. He had a complete clinical recovery with no long-term sequela. [Table 3] lists the summary of complications.
| Type | Complication | Tumor size (cm) | Location | Ablation modality | Embolization modality |
|---|---|---|---|---|---|
| Minor | Perirenal hematoma and hematuria | 4.5 | Central | Cryoablation | Ethanol/ethiodized oil |
| Minor | Perirenal hematoma | 5.4 | Exophytic | Cryoablation | Ethanol/ethiodized oil |
| Minor | Hematuria | 2.5 | Central | Cryoablation | Ethanol/absorbable gelatin sponge |
| Major | Ureteropelvic stricture and perirenal abscess | 4.5 | Central | Cryoablation | Ethanol/ethiodized oil |
Survival outcomes
The overall survival was estimated at 58.6% at 5 years. Some early cohort patients were deceased from causes unrelated to the primary renal tumors, including heart disease and other malignancies. Overall survival and time until recurrence curves are shown in [Figures 2 and 3].
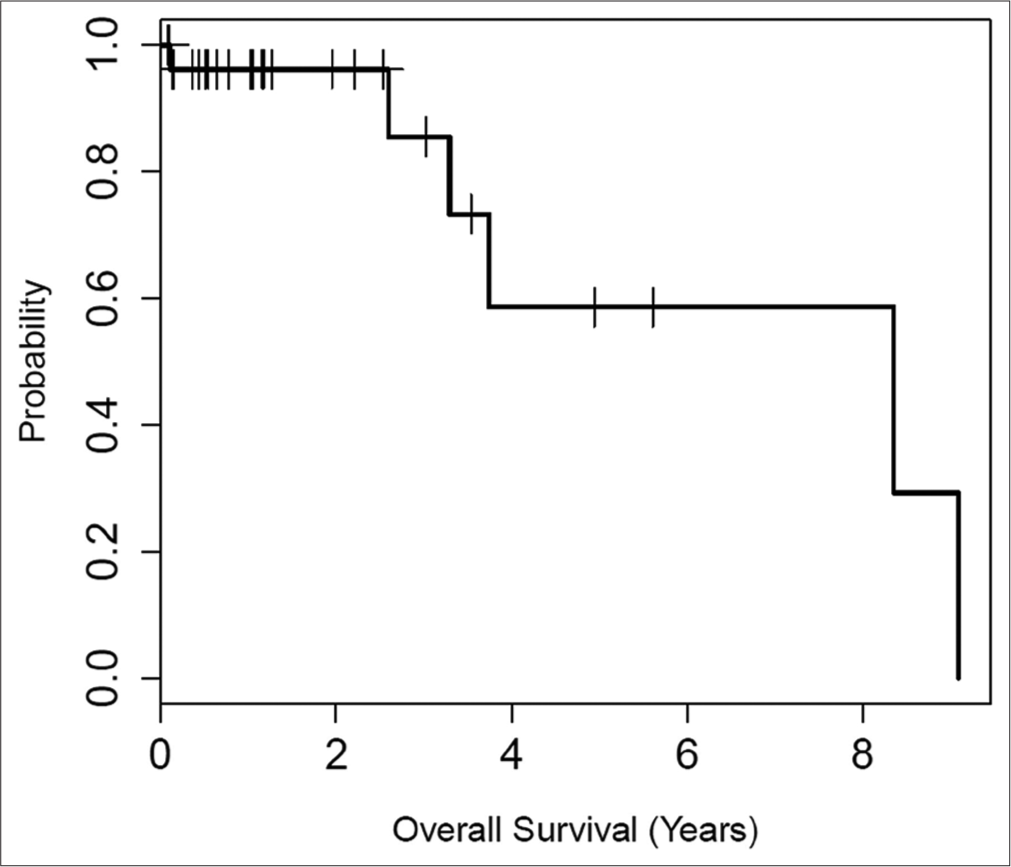
- Kaplan–Meier plot of overall survival for patients with T1B or central renal tumors treated with combined embolization and thermal ablation. The median survival was 8.4 years. At 5 years, the overall survival estimate was 58.6%, with a corresponding 95% confidence interval of (32.6%, 100%). The overall survival reflects our elderly patient population. No death related to primary renal tumors was observed.

- Kaplan–Meier plot of time until tumor recurrence for patients with T1B or central renal tumors treated with combined embolization and thermal ablation. At 5 years, the TTP estimate was 90%, with a corresponding 95% confidence interval of 77.5%, 100%.
DISCUSSION
RFA and CA have become widely accepted for the treatment of T1a renal tumors. Recommendations for using these techniques have been incorporated into the guideline of the American Urological Association.[7,8] These guidelines and the current literature indicate that T1b and centrally located renal tumors treated with thermal ablation have higher risks of local recurrence and complications.[13-15] Thus, surgical resection remains the standard of care for T1b renal tumors. However, partial nephrectomy for T1b renal tumors is associated with higher local recurrence and complication rates. Joniau et al., in a cohort of 67 patients with T1b renal masses treated with partial nephrectomy, reported tumor recurrence in 7 patients (10.4%). In this cohort, 4 patients (6%) developed perirenal hematoma with two patients requiring embolization or surgery, 2 patients (3%) needed hemodialysis, and 1 patient (1.5%) died due to hypovolemic shock.[23] A literature review shows a local recurrence rate of 0.5–9.8% and a post-operative complication rate of 8.4–41.5% for patients with T1b RCC undergoing partial nephrectomy. The outcomes were comparable with radical nephrectomy, but partial nephrectomy has better preservation of renal function.[24] Hasegawa et al. compared CA and RFA to treat 46 patients with T1b RCCs. Following successful treatment, tumor recurrence was observed in 3 of 21 patients (14%) in the RFA group and in 2 patients (9%) in the CA group.[25] Atwell et al. treated 46 patients with T1b RCC with CA, and they performed TAE embolization before the ablation in seven patients with highly vascular tumors larger than 5 cm. In this cohort, technical success was achieved in 45 of 46 patients (97.2%), and local tumor recurrence was seen in one patient. They also reported 7 major complications (15.2%), including bleeding requiring embolization or transfusion, in four patients.[26] Arima et al. published the largest cohort of patients with RCC treated with a combination of TAE and RFA, including 31 patients with 36 tumors (30 cT1a and six cT1b tumors). The tumor recurrence rate after successful RFA was 3% (1/31). Three patients had post-operative bleeding (9%) and one developed procedure site abscess (3%).[16]
The current cohort presents the largest number of patients with T1b RCC (n = 24) treated with a combination of ablation and embolization. The combined technique allowed for the successful treatment of all patients in a single ablation session. The mechanisms contributing to these results likely include a marked reduction in vascularity of the tumor after TAE, which decreases the thermal sink effect and increases the effectiveness of ablation. In addition, TAE facilitates the delineation of the tumor and contributes to ischemic tissue necrosis. Embolizing large and central renal masses before ablation may also decrease the procedural bleeding.[16,18,27] In the present cohort, ethanol was the primary embolization agent in 25 of 28 patients. Renal artery embolization with ethanol has been used for palliative treatment for RCC, for the treatment of arterial-venous malformations, and renal angiomyolipomas.[28,29] Ethanol causes vascular occlusion due to small artery spasms, endothelial damage, sludging of erythrocytes in small arteries, and perivascular necrosis.[30]
The three patients who presented perirenal hematoma in this study had self-limiting bleeding, and no transfusion or new embolization was needed. Finally, the local recurrence rate in this study is comparable to the local recurrence rate in partial nephrectomy series.[31] The overall survival curve reflects this elderly patient population with multiple comorbidities. The tumors found in the follow-up imaging for the two patients with recurrences were technically suitable for retreatment with percutaneous ablation; however, repeat ablation was not warranted because of the patients’ advanced age and comorbidities.
The present study has several limitations. Histological confirmation was missing for two patients with imaging consistent with RCC. A variety of embolization techniques was used per operator discretion; however, all the embolization techniques used are expected to decrease tumor vascularity and the thermal sink effect. Due to the retrospective single-arm design, no comparison group treated with ablation alone or partial nephrectomy is available. It is not possible to determine if the TAE before ablation leads to improved outcomes and decreased recurrence in comparison to ablation alone without a prospective randomized cohort. Several operators performed the procedures, which are expected in a cohort patient evaluating procedures performed over 11 years, regardless excellent tumor control was achieved. Conventionally, thermal ablation has been reserved for elderly patients with multiple comorbidities which preclude surgery. This inherent difference makes a comparison of thermal ablation and nephrectomy cohorts difficult.
CONCLUSION
In this cohort of patients, a combination of transarterial renal embolization and percutaneous CT-guided renal ablation was an effective, well-tolerated, and safe treatment for patients with T1b and central tumors in patients with high surgical risk. For patients suitable for surgical treatment, prospective studies are needed to direct compare TAE plus percutaneous ablation versus percutaneous ablation only and determine if these techniques have the same oncologic effectiveness as partial nephrectomy and if a minimally invasive percutaneous approach can reduce surgical morbidity associated with partial nephrectomy.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-49.
- [CrossRef] [PubMed] [Google Scholar]
- Hereditary kidney cancer syndromes. Adv Chronic Kidney Dis. 2014;21:81-90.
- [CrossRef] [PubMed] [Google Scholar]
- Understanding pathologic variants of renal cell carcinoma: Distilling therapeutic opportunities from biologic complexity. Eur Urol. 2015;67:85-97.
- [CrossRef] [PubMed] [Google Scholar]
- Management of renal masses and localized renal cancer: Systematic review and meta-analysis. J Urol. 2016;196:989-99.
- [CrossRef] [PubMed] [Google Scholar]
- European association of urology guidelines on renal cell carcinoma: The 2019 update. Eur Urol. 2019;75:799-810.
- [CrossRef] [PubMed] [Google Scholar]
- Renal mass and localized renal cancer: AUA guideline. J Urol. 2017;198:520-9.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term clinical outcomes following radiofrequency and microwave ablation of renal cell carcinoma at a single VA medical center. Curr Probl Diagn Radiol. 2018;47:98-102.
- [CrossRef] [PubMed] [Google Scholar]
- Long-term oncologic outcomes after radiofrequency ablation for T1 renal cell carcinoma. Eur Urol. 2013;63:486-92.
- [CrossRef] [PubMed] [Google Scholar]
- Midterm results of radiofrequency ablation versus nephrectomy for T1a renal cell carcinoma. Jpn J Radiol. 2010;28:460-8.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous image-guided thermal ablation for multifocal renal cell carcinoma: 10-year experience at a single center. AJR Am J Roentgenol. 2017;209:733-9.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness and safety of computed tomography-guided radiofrequency ablation of renal cancer: A 14-year single institution experience in 203 patients. Eur Radiol. 2016;26:1656-64.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of the RENAL and mRENAL scores and the relative importance of their components in the prediction of complications and local progression after percutaneous renal cryoablation. J Vasc Interv Radiol. 2017;28:860-7.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous radiofrequency ablation for renal cell carcinoma vs. partial nephrectomy: Comparison of long-term oncologic outcomes in both clear cell and non-clear cell of the most common subtype. Urol Oncol. 2017;35:530.e1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous radiofrequency ablation with transarterial embolization is useful for treatment of stage 1 renal cell carcinoma with surgical risk: Results at 2-year mean follow up. Int J Urol. 2007;14:585-90.
- [CrossRef] [PubMed] [Google Scholar]
- Sequential combination treatment (arterial embolization and percutaneous radiofrequency ablation) of inoperable renal cell carcinoma: Single-center pilot study. Acta Radiol. 2012;53:410-4.
- [CrossRef] [PubMed] [Google Scholar]
- Role of combined embolization and ablation in management of renal masses. Semin Intervent Radiol. 2014;31:82-5.
- [CrossRef] [PubMed] [Google Scholar]
- Trans-arterial embolization of renal cell carcinoma prior to percutaneous ablation: Technical aspects, institutional experience, and brief review of the literature. Curr Urol. 2018;12:43-9.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous radiofrequency ablation of renal tumors: Technique, complications, and outcomes. J Vasc Interv Radiol. 2005;16:679-88.
- [CrossRef] [PubMed] [Google Scholar]
- Residual and recurrent disease following renal energy ablative therapy: A multi-institutional study. J Urol. 2006;176:1973-7.
- [CrossRef] [PubMed] [Google Scholar]
- Radio-frequency ablation of renal cell carcinoma: Early clinical experience. Radiology. 2000;217:665-72.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome of nephron-sparing surgery for T1b renal cell carcinoma. BJU Int. 2009;103:1344-8.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment outcomes of partial nephrectomy for T1b tumours. Curr Opin Urol. 2013;23:403-10.
- [CrossRef] [PubMed] [Google Scholar]
- Radiofrequency ablation versus cryoablation for T1b renal cell carcinoma: A multi-center study. Jpn J Radiol. 2018;36:551-8.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous cryoablation of stage T1b renal cell carcinoma: Technique considerations, safety, and local tumor control. J Vasc Interv Radiol. 2015;26:792-9.
- [CrossRef] [PubMed] [Google Scholar]
- Radiofrequency ablation combined with renal arterial embolization for the treatment of unresectable renal cell carcinoma larger than 3.5 cm: Initial experience. Cardiovasc Intervent Radiol. 2006;29:389-94.
- [CrossRef] [PubMed] [Google Scholar]
- Transarterial ethanol ablation for sporadic and nonhemorrhaging angiomyolipoma in the kidney. Eur J Radiol. 2009;72:139-45.
- [CrossRef] [PubMed] [Google Scholar]
- Ethanol ablation of renal cell carcinoma for palliation of symptoms in advanced disease. J Palliat Med. 2010;13:117-20.
- [CrossRef] [PubMed] [Google Scholar]
- Early mechanism of action of arterially infused alcohol U.S.P. in renal devitalization. Radiology. 1982;145:45-8.
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for postoperative hemorrhage after partial nephrectomy. Korean J Urol. 2014;55:17-22.
- [CrossRef] [PubMed] [Google Scholar]







