Translate this page into:
Arterial enhancement on triphasic computed tomography scan predicts response of colorectal liver metastases to chemoembolization: A case–control study

*Corresponding author: Michael Moser, Department of Surgery, University of Saskatchewan, Saskatoon, Canada. mike.moser@usask.ca
-
Received: ,
Accepted: ,
How to cite this article: Wall C, Amiraslany A, Ahmed S, Moser M. Arterial enhancement on triphasic computed tomography scan predicts response of colorectal liver metastases to chemoembolization: A case–control study. Am J Interv Radiol 2023;7:13.
Abstract
Objectives:
The arterial enhancement fraction (AEF), a simple calculation based on a standard triple-phase computed tomography (CT) scan, has been shown to predict treatment response in radioembolization of colorectal liver metastases (CRLM). The current study aims to determine if arterial enhancement also predicts treatment response in transarterial chemoembolization (TACE) of CRLM, which uses a larger particle size and exerts an ischemic effect.
Materials and Methods:
A retrospective analysis of our experience with TACE for CRLM between 2013 and 2022 yielded 97 TACE treatments for CRLM. The study included the first TACE treatment patients having a triple-phase CT scan before and after TACE, yielding 62 tumors treated with TACE of irinotecan drug-eluting beads in 36 patients. Tumors with complete response or partial response based on CT-based modified RECIST criteria were considered to be “responders,” whereas tumors that had progressive disease or stable disease were considered to be “non-responders.”
Results:
The responders differed from the non-responders in terms of arterial phase enhancement (APhE) (9.5 [interquartile range, IQR 6, 17] vs. 2 [IQR 1, 5] Hounsfield units [HUs], P < 0.001) and AEF (0.7 [IQR 0.5, 1] vs. 0.3 [IQR 0.1, 1], P = 0.01), both validated measures of hepatic arterial perfusion. Receiver operating characteristic curve analysis yielded a 5.5 HU cutoff for APhE. Those tumors with APhE >5.5 HU had a response rate of 72%, whereas those <5.5 HU had a response rate of 21%. Median overall survival for patients with tumors having APhE >5.5 HU was 22.4 months (IQR 13, 32) versus 14.5 months (IQR 10, 19) for those with APhE ≤5.5 HU, but this did not achieve statistical significance (P = 0.14).
Conclusion:
CRLM with greater hepatic arterial blood supply as measured by the APhE and AEF have a higher probability of TACE treatment response.
Keywords
Arterial enhancement
Arterial perfusion
Chemoembolization
Colorectal liver metastasis
Computed tomography scan
INTRODUCTION
Transarterial chemoembolization (TACE) has a defined role in the management of intermediate-stage hepatocellular carcinoma (HCC), whereas its role in colorectal liver metastasis (CRLM) is evolving.[1,2] Although it has been traditionally used in unresectable CRLM, TACE is increasingly used with microwave ablation or for downsizing before surgical resection.[3] Predicting which tumors have a better chance of responding to TACE would help choose between the treatments available for CRLM.
It has previously been shown that a higher arterial enhancement fraction (AEF) in CRLM predicted a better response to radioembolization.[4] Chemoembolization and radioembolization have similarities, but there are also significant differences in terms of particle size and mechanism of action. To test our hypothesis that better arterial characteristics in CRLM on computed tomography (CT) scans would result in a better response to TACE treatment, we performed a retrospective case–control study similar to that of Boas et al.
MATERIALS AND METHODS
All TACE procedures performed for a diagnosis of CRLM between 2013 and 2020 at our tertiary care teaching hospital were considered for this study. The administrative database used to identify the study population started in 2013. We wished to ensure at least a 2-year follow-up, so the period was chosen from May 1, 2013, to December 2020. Our local institutional ethics board reviewed and approved this study (#1893).
The study aimed to determine whether arterial enhancement characteristics in individual tumors would predict response to TACE. Because the three validated measures of hepatic arterial blood flow require data from the unenhanced, arterial, and venous phases of the CT scan, only those cases where a triple-phase CT scan had been obtained before TACE treatment and for follow-up were included in the study. As in a prior study involving radioembolization, we selected the two largest CRLMs in each of the remaining cases to form our study population.[4]
Pretreatment scans were obtained no more than 4 weeks before treatment using our standard protocol. Isovue (Bracco Diagnostics, Princeton, NJ) 100 cc injection was performed at 4 cc/s. The arterial phase scan was obtained 5 s after the aorta at the level of the celiac artery attained an attenuation of 120 Hounsfield units (HUs), while the venous phase was obtained 70 s after contrast injection.
TACE treatments were performed after cannulation of the right or left hepatic artery with a Glidecath® hydrophilic coated catheter (Terumo, Tokyo, Japan), with PROGREAT® microcatheter (Terumo, Tokyo, Japan) used for selective cannulation. In all cases, 100 mg of DEBIRI™ Irinotecan Drug-Eluting Beads (Boston Scientific, Marlborough, MA) was injected. Patients with bilobar disease underwent TACE treatment of one side of the liver, followed within 10–14 days by treatment of the contralateral side.
Follow-up triple-phase CT scan imaging was performed 8–12 weeks after TACE treatment using the same protocol as for the pretreatment scan. Using tools in the Picture Archiving and Communication System (Phillips, Amsterdam), the average attenuation, measured in HU of a circle covering at least 80% of the tumor, the aorta, and the portal vein, were measured on the pretreatment scan in approximately the same location for each of the non-contrasted, arterial, and portal venous phases. Three validated CT-based arterial perfusion measures were calculated for each tumor [Table 1]: The hepatic artery coefficient, the AEF, and the arterial phase enhancement (APhE).[4]
| Measure of vascularity | Calculation |
|---|---|
| Arterial phase enhancement | X2-X1 |
| Arterial enhancement fraction | |
| Hepatic artery coefficient |
a1, a2, a3 represent the mean HUs measurement in the aorta in the unenhanced, arterial and portal phases, respectively; v1, v2, v3 represent the mean HUs measurement in the portal vein in the unenhanced, arterial and portal phases, respectively; and x1, x2, x3 represent the mean HUs measurement in the tumor in the unenhanced, arterial and portal phases, respectively. APhE: Arterial phase enhancement, AEF: Arterial enhancement fraction, HAC: Hepatic artery coefficient, HUs: Hounsfield units, CT: Computed tomography
Treatment response was determined using the validated modified RECIST CT-based criteria [Table 2], which considers only objective measures of both size and enhancement values.[5] Treatment response can, therefore, be calculated using a spreadsheet based on a few numerical inputs without subjectivity or observer variation. Complete Response (CR) or partial Response (PR) formed the definition of a “responder” to TACE, and Stable Disease (SD) or Progressive Disease (PD) formed the definition of a “non-responder.” Mann–Whitney U-test was used for between-group comparisons of arterial perfusion indices, given that a normal distribution could not be assumed for the indices that are fractions. Median overall survival was defined as the survival from the time of the first TACE treatment and calculated using Kaplan–Meier survival analysis. Mann–Whitney U-test, Chi-squared test, and Kaplan–Meier survival analysis were calculated using SPSS 28 (IBM Corporation, Armonk, NY). A P = 0.05 was considered statistically significant in all cases.
| Abbreviation | Response | Definition |
|---|---|---|
| CR | Complete response | Disappearance of all lesions |
| PR | Partial response | ≥10% decrease in target lesion size or ≥15% decrease in tumor density |
| SD | Stabledisease | None of CR, PR, or PD criteria met |
| PD | Progressive disease | ≥10% increase in target lesion size and Does not meet tumor density criteria of PR |
Based on Chung WS, Park MS, Shin SJ, Baek SE, Kim YE, Choi JY, et al. Response evaluation in patients with colorectal liver metastases: RECIST version 1.1 versus modified CT criteria. AJR Am J Roentgenol 2012;199:809-15. RECIST: Response evaluation criteria in solid tumors, CT: Computed tomography
RESULTS
Between 2013 and 2022, our institution performed a total of 212 TACE procedures; the indication for TACE was CRLM for 97 of these procedures [Figure 1]. In 4 cases, the CRLM was resected surgically, and no follow-up imaging was performed; these were excluded from further analysis. The resulting 62 CRLM formed the study population, all of which had not received prior TACE (nor TARE) and had undergone pretreatment and post-treatment triple-phase CT scan. One patient had undergone microwave ablation for metastasis in the left liver, which proved effective, and subsequently underwent selective right-sided TACE for further metastases about 2 years later. As such, the metastasis in this patient that was included in our TACE study had not previously been ablated. None of the patients in this study had undergone hepatic external beam radiation.

- Patient population selected for this study from 97 TACE procedures performed at a single institution between 2013 and 2020. CRLM: Colorectal liver metastases, TACE: Transarterial chemoembolization.
The demographics of the 36 patients are shown in Table 3. The characteristics of the 62 tumors in these 36 patients are shown in Table 4. The responder group did not differ significantly from the non-responder group in terms of tumor diameter, whether the CRLM was solitary or multiple or had undergone treatment with bevacizumab. PR was noted for 28 tumors, SD for 13 tumors, and PD for 21; no complete responses were observed.
| Age (years, IQR) | 62 (47, 68) |
| Sex (M:F) | 29:7 |
| Location of primary | |
| Rectum | 16 (44%) |
| Colon | 20 (56%) |
| Synchronous liver metastases at the time of diagnosis | 23 (64%) |
| Solitary liver metastasis | 9 (25%) |
| Primary in situ at the time of TACE | 14 (39%) |
| Chemotherapy before TACE | |
| FOLFIRI | 17 (47%) |
| FOLFOX | 7 (19%) |
| FOLFOXIRI | 5 (14%) |
| Capecitabine | 3 (8%) |
| Capecitabine/Irinotecan | 1 (3%) |
| None | 3 (8%) |
| Cycles before TACE (median, IQR) | 12 (10, 15) |
| Received bevacizumab before TACE | 25 (69%) |
| Time from diagnosis of liver | |
| metastasis to TACE (months, IQR) | 12.2 (6.6, 19.4) |
| TACE treatment | |
| Irinotecan drug eluting beads | 36 (100%) |
| Selective | 5 (14%) |
| Nonselective | 31 (86%) |
IQR: Inter quartile range, TACE: Transarterial chemoembolization
| Responder (n=28) | Non-responder (n=34) | P-value | |
|---|---|---|---|
| Median tumor diameter (cm, IQR) | 4.6 (2.1, 8.7) | 5.6 (3.8, 8.0) | 0.28* |
| Solitary liver metastasis | 3 (11%) | 6 (17%) | 0.44† |
| Prior bevacizumab | 19 (68%) | 22 (65%) | 0.79† |
| Arterial phase enhancement (IQR) | 9.5 (5.5, 17.3) | 2 (1, 4.8) | <0.0001* |
| Arterial enhancement fraction (IQR) | 0.7 (0.5, 1) | 0.3 (0.1, 1) | 0.01* |
| Hepatic artery coefficient (IQR) | 0 (−0.1, 0) | 0 (−0.1, 0) | 0.41* |
IQR: Inter quartile range, TACE: Transarterial chemoembolization. Non-responder: StableDisease or Progressive Disease as per modified CT-based RECIST criteria. Responder: Partial response as per modified CT-based RECIST criteria. *Calculated using the Mann–Whitney U test. †Calculated using the Chi-squared test. Bold values denote P value < 0.05.
Of the three measures of hepatic arterial blood supply, APhE yielded the most significant (P < 0.0001). The AEF was also different between the two groups (P = 0.01).
Receiver operating characteristic analysis [Figure 2] of APhE in predicting treatment response revealed an area under the curve (AUC) of 0.83, with optimal discrimination identified at an APhE of 5.5 HU, yielding a sensitivity of 0.75 and 1-specificity of 0.219. The same calculations for AEF yielded an AUC of 0.69, with optimal discrimination identified at 0.4, the same cutoff determined for AEF in a similar paper predicting response to radioembolization.[4]
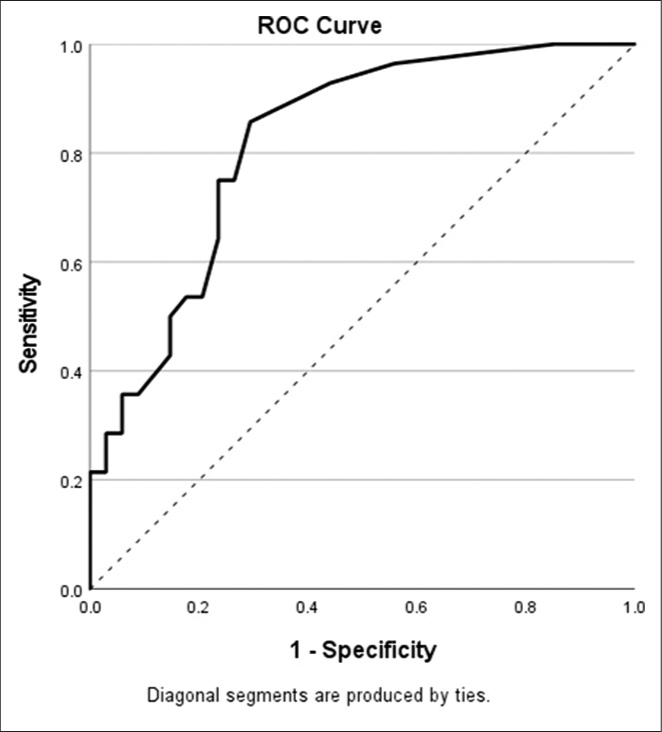
- ROC curve for the arterial phase enhancement as a predictor of response of CRLM to TACE. CRLM: Colorectal liver metastases, ROC: Receiver operating characteristic, TACE: Transarterial chemoembolization.
The median overall survival for patients having their solitary or both of their two largest tumors having an APhE of >5.5 HU was compared to those that did not [Figure 3]. Survival appeared to be slightly better for the former group, with a median overall survival of 22.4 months interquartile range (IQR 13, 32), compared to 14.5 months (IQR 10, 19) for the latter group, but this did not achieve statistical significance (P = 0.14 by Log-Rank test).
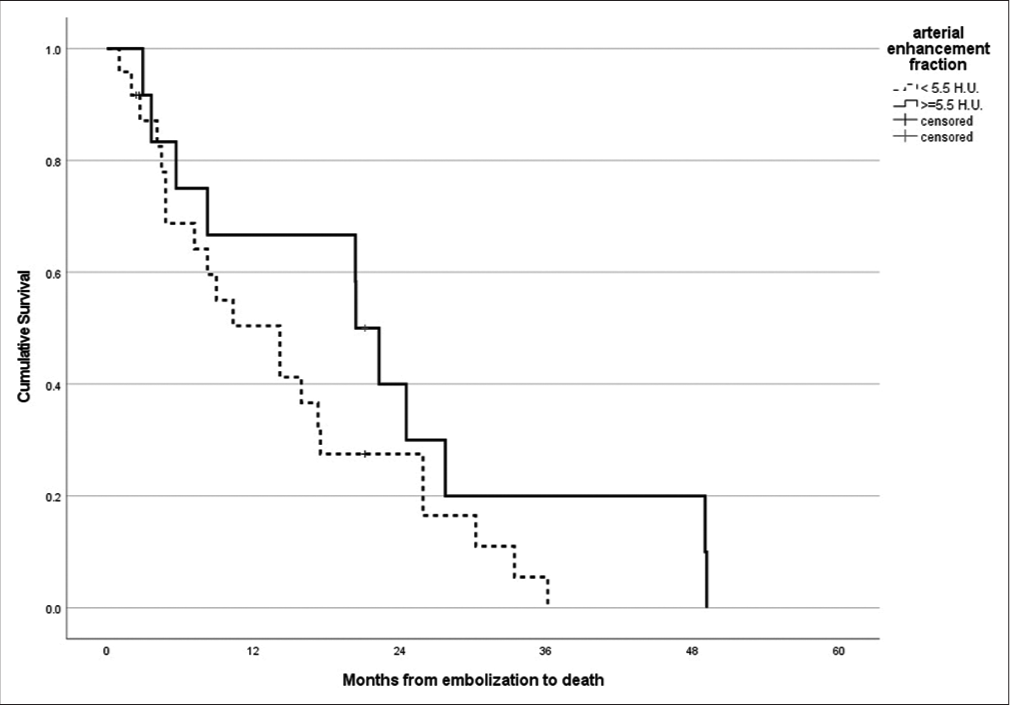
- Kaplan–Meier survival curve and survival estimation for patients with the solitary or both of the two largest CRLM having arterial phase enhancement of ≥5.5 HU (solid line) versus those that did not (dashed line). CRLM: Colorectal liver metastases.
DISCUSSION
Our results suggest that TACE for “more arterial” CRLM on CT scan is more likely to lead to a treatment response than for “less arterial” CRLM, as measured by AEF and the simpler APhE.
Another study of patients undergoing radioembolization made a similar observation: the AEF was a significant predictor of treatment response. Indeed, the mean or median values of AEF between responders and non-responders in the two studies were nearly identical (~0.7 for the responders and ~0.3 for the non-responders).[4] In our study, we found that an even simpler measure of arterial perfusion, the APhE, also predicted a response to TACE treatment and that this appeared to be a more significant predictor. APhE is calculated simply as the difference between the attenuation of the tumor during the arterial phase minus the attenuation during the uncontrasted phase of a triple-phase CT scan. In our multidisciplinary rounds, we already use APhE without needing a calculator or an app to assess the chance of treatment response to TACE.
Complex imaging and computer analyses can provide more specific and detailed information about the arterial blood supply to tumors, generally for research purposes.[6] However, we wished to arrive at a simple and clinically useful means of predicting treatment response from non-specialized scans, such as the triple-phase CT scan.
Radioembolization and chemoembolization both involve injecting microbeads into hepatic arteries, but they differ in the size of the microbeads used. This difference may explain why the arterial enhancement measures that predict response to radioembolization are different from those for chemoembolization. TACE beads measure between 70 and 300 microns, whereas Y-90 beads used in radioembolization measure between 20 and 30 microns. TACE microbeads deliver high concentrations of chemotherapy to the tumor and also block small arterioles,[7] whereas Y-90 microspheres deliver radiation to the tumor and the area around the tumor with no or minimal occlusive effect.[8]
Another study documented a correlation between arterial perfusion of CRLM and response to chemotherapy, suggesting that arterial perfusion is important for delivering intravenous chemotherapy to the tumor.[9] Our study and the similar study looking at radioembolization leave it unclear whether the observed improvement in treatment response for tumors with greater arterial perfusion is due to the increased delivery of microbeads,[4] or whether the tumors’ survival requires increased vascularity for survival, which is reduced or eliminated with TACE. The results of the radioembolization study suggest that the increased treatment effect may be due to greater bead delivery, given that the ischemic effect is thought to be minimal.
The benefit of the chemotherapy versus the ischemic effect of the microbeads in TACE has recently been questioned, at least in studies looking at TACE and bland embolization for HCC, which show similar results whether the beads are loaded with chemotherapy or not (bland embolization).[10,11] On the other hand, the lack of significance of the APhE in one study but not the other may be simply related to sample size, one of the limitations of both the current study and the one by Boas et al.
Although TACE is generally regarded as safe, there have been instances of liver failure or inadvertent embolization of other organs.[12] Prior knowledge of a patient’s potential response to TACE can assist in determining if the benefits of the treatment outweigh the risks. As TACE is being used more frequently in preparation for other procedures, such as microwave ablation and surgical resection, knowledge of the tumors’ arterial blood supply may assist tumor boards in making decisions about the use of TACE before these procedures.
The obvious limitations of our study include the small sample size and the retrospective design; nonetheless, the results were significant. Because TACE microbeads are large enough to cause significant ischemia in tumor vessels, we excluded those cases in patients with previous TACE treatments. Calculating the APhE requires a triple-phase CT scan, but in more than half the cases, this scan was not performed. We are currently discussing ways to change this practice at our institution. It is hoped that the relatively small added cost of the triple versus the single-phase CT scan is outweighed by the added information obtained for planning TACE procedures. The measures of arterial enhancement should be feasible on any triple-phase CT scan, and the methodology for TACE is relatively standard from center to center, meaning that these results should be generalizable to most other centers performing TACE.
CONCLUSION
Our findings are in line with existing research on radioembolization, which has demonstrated that CRLM with increased hepatic arterial blood flow exhibits a more robust response to TACE. The calculation of both APhE and AEF is a straightforward process. Larger prospective studies with triple-phase CT scans before and after all TACE procedures are warranted.
Authors’ contributions
CW, AA, MM: Study design. AA, MM: Data acquisition. AA, MM: Data analysis/interpretation. MM: Statistical analysis. CW, AA, SA, and MM: Drafting and revising the article. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using the AI.
Financial support and sponsorship
Nil.
References
- A review and update of treatment options and controversies in the management of hepatocellular carcinoma. Ann Surg. 2016;263:1112-25.
- [CrossRef] [PubMed] [Google Scholar]
- Chemoembolization in colorectal liver metastases: The rebirth. Anticancer Res. 2014;34:575-84.
- [Google Scholar]
- Comparison of combined transarterial chemoembolization and ablations in patients with hepatocellular carcinoma: A systematic review and meta-analysis. Abdom Radiol (NY). 2022;47:1009-23.
- [CrossRef] [PubMed] [Google Scholar]
- Quantitative measurements of enhancement on preprocedure triphasic CT can predict response of colorectal liver metastases to radioembolization. AJR Am J Roentgenol. 2016;207:671-5.
- [CrossRef] [PubMed] [Google Scholar]
- Response evaluation in patients with colorectal liver metastases: RECIST version 1.1 versus modified CT criteria. AJR Am J Roentgenol. 2012;199:809-15.
- [CrossRef] [PubMed] [Google Scholar]
- Can cone-beam CT tumor blood volume predict the response to chemoembolization of colorectal liver metastases? Results of an observational study. Eur Radiol. 2019;29:5022-31.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacokinetic and safety study of doxorubicin-eluting beads in a porcine model of hepatic arterial embolization. J Vasc Interv Radiol. 2006;17:1335-43.
- [CrossRef] [PubMed] [Google Scholar]
- Side effects of yttrium-90 radioembolization. Front Oncol. 2014;4:198.
- [CrossRef] [PubMed] [Google Scholar]
- Liver metastases on quantitative color mapping of the arterial enhancement fraction from multiphasic CT scans: Evaluation of the hemodynamic features and correlation with the chemotherapy response. Eur J Radiol. 2011;80:e278-83.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of trans-arterial chemoembolization and bland embolization for the treatment of hepatocellular carcinoma: A propensity score analysis. Cancers (Basel). 2021;13:812.
- [CrossRef] [PubMed] [Google Scholar]
- Transarterial bland versus chemoembolization for hepatocellular carcinoma: Rethinking a gold standard. J Surg Res. 2016;200:552-9.
- [CrossRef] [PubMed] [Google Scholar]
- Chemoembolization of hepatic neoplasms: Safety, complications, and when to worry. Radiographics. 1999;19:399-414.
- [CrossRef] [PubMed] [Google Scholar]







