Translate this page into:
A History of Mobile Stroke Units and Review of Literature

Corresponding Author: Thomas Pieters, Department of Neurosurgery, University of Rochester, 601 Elmwood Avenue, Rochester, NY, 14642, United States. E-mail: thomasa_pieters@urmc.rochester.edu
-
Received: ,
Accepted: ,
How to cite this article: Towner J, Pieters T, Schmidt T, Pilcher W, Bhalla T. A History of Mobile Stroke Units and Review of Literature. Am J Interv Radiol 2018, 2(9) 1-5.
Abstract
Using intravenous tissue plasminogen activator (IV tPA), improved functional outcomes are seen with earlier initiation of treatment. Recent studies have shown endovascular revascularization to be a revolutionary and effective treatment. There have been many initiatives focused on improving public education and awareness of stroke symptoms. The concept of a mobile stroke unit (MSU) was created as a way of bringing treatment to patients. Earlier CT scans, delivery of tPA, proper triage and on-scene goal-directed care were the primary goals with these units. It was thought that rapid implementation would shorten hospital stay and improve outcomes. The University of Saarland found a decrease of 41 minutes from stroke alarm to therapeutic decision when an MSU was used. A second trial found a decrease of 25 minutes in time to treatment, an increase in the rate of thrombolysis utilization, and no change in the rates of intracranial hemorrhage or 7-day mortality when an MSU was employed. In 2016, a Lancet article showed that 3 month modified Rankin Scale (mRS) and 3-month mortality were improved in MSU patients. Finally, starting thrombolytic therapy in the MSU was associated with higher probability of mRS of 0-3 but not an improved 3-month survival rate. Long-term results are thus far not available precluding an effective cost-benefit analysis. Many study results are not generalizable as they compare a single hospital system and specialized MSU team to conventional care delivered by a multiple healthcare systems. Future studies will target these limitations.
Keywords
Infarct
Ischemic
Mobile stroke
Tissue plasminogen activator
INTRODUCTION
Nearly 87% of the more than 795,000 yearly strokes in the United States are ischemic infarcts.[1] Over 130,000, or 1 in 20, deaths in the United States are attributable to stroke.[1] Of the patients who survive their stroke, many have significant disabilities resulting in an annual 33 billion dollars in direct and indirect costs related to strokes. Despite these seemingly discouraging figures, when historically framed, the incidence and mortality of stroke are actually declining.[1] Treatment of ischemic infarcts has historically been restricted to supportive measures with the majority of innovations, research, and treatment focused on prevention. In 1995, the National Institute of Neurological Disorders and Stroke released their landmark manuscript regarding intravenous tissue plasminogen activator (IV tPA), (Our manufacturer: Genentech, San Francisco, California) for acute ischemic stroke.[2] Since that time, many trials have confirmed IV tPA is an effective treatment for acute ischemic stroke. A pooled analysis of 9 trials examining IV tPA for acute ischemic stroke shows that there are improved functional outcomes when treatment occurs within 4.5 h of stroke onset and treatment benefits are greater with earlier initiation of treatment.[3] Recent studies have shown endovascular revascularization to be an effective treatment for patients with acute ischemic strokes ushering a new era in stroke care.[4-9]
Challenge
Despite the recent, remarkable paradigm shift toward readily available effective stroke treatment in the form of tPA or endovascular as opposed to the historical standard of supportive care with a focus on primary prevention, the vast majority of those affected by strokes still receive no acute treatment. The window of benefit for medical therapy in the form of tPA for all patients closes 4.5 h from symptom onset. The time-frame of benefit for endovascular revascularization is not as clearly defined and continues to expand, but there is decreasing benefit further from ictus. The axiom “time is brain” refers to the approximately 2 million neurons lost every minute in an ischemic stroke.[10] As time passes from ictus, the less neuronal tissue is salvageable with recanalization of the occluded vessel, and more is risked, as hemorrhage-prone “dead brain” accumulates. The number needed to treat for a modified Rankin Scale (mRS) of 0 or 1 is 4.5 for patients treated with IV tPA at <1.5 h from ictus, increases to 9 for those treated between 1.5 and 3 h, and is 14.1 for those treated between 3 and 4.5 h.[11] The time until stroke treatment can be initiated is dependent on the patient, family, and bystander recognition and action, pre-hospital care, and hospital care. Only 11.3% of patients receive IV tPA within 1.5 of stroke onset.[12] There have been many initiatives focused on improving public education and awareness of stroke symptoms, as well as placing emphasis on the emergent nature of seeking evaluation in an effort to decrease time to presentation.[13,14]
The Get With The Guidelines-Stroke registry found that less than one-third of treatment-eligible patients with ischemic strokes receive IV tPA within 60 min of arriving at the hospital.[15] Once patients arrive at the hospital, appropriate workup, and treatment must include a National Institute of Health Stroke Scale, assessment for any IV tPA contraindications, treatment of any reversible contraindications (i.e., hypertension), and head imaging to rule out any intracerebral hemorrhage. There are many obstacles to completing these tasks within 60 min, but with the help of best practice guidelines, valuable minutes can be saved. When 10 best practice guidelines are implemented, there is a 10-min decrease in the median door-to-needle time.[16]
RATIONALE/COMPONENTS
The concept of a mobile stroke unit (MSU) was first published by Fassbender et al. in 2003 as a way of “bringing treatment to the patient rather than the patient to the treatment.”[17]
All MSUs have the basic components to provide assessment and treatment of acute ischemic infarcts, including standard ambulance equipment and medications, a computed tomography (CT) scanner, point-of-care laboratory equipment, telemedicine capabilities, and, of course, tPA. A typical MSU interior can be seen in (Figure 1). In addition to the staff of a standard ambulance, the unit must also have a physician, either in person or through telemedicine, and a member trained, either primarily or cross-trained, as a CT technologist. In the first generation of MSUs, standard ambulances could not house all the necessary components, but miniaturization of technology has now allowed all the necessary components to fit into a standard ambulance. These team members work to quickly and efficiently diagnose or rule-out stroke and determine IV tPA eligibility. The entire team’s sole focus is on the patient being evaluated with all the competing variables of in-hospital care removed from the equation. Instances where delays may arise, due to triage of multiple patients, competing obligations of the hospitalist, availability of a CT scanner, or attention of emergency department nurses, and technicians are effectively eliminated when the hospital is brought to the patient.
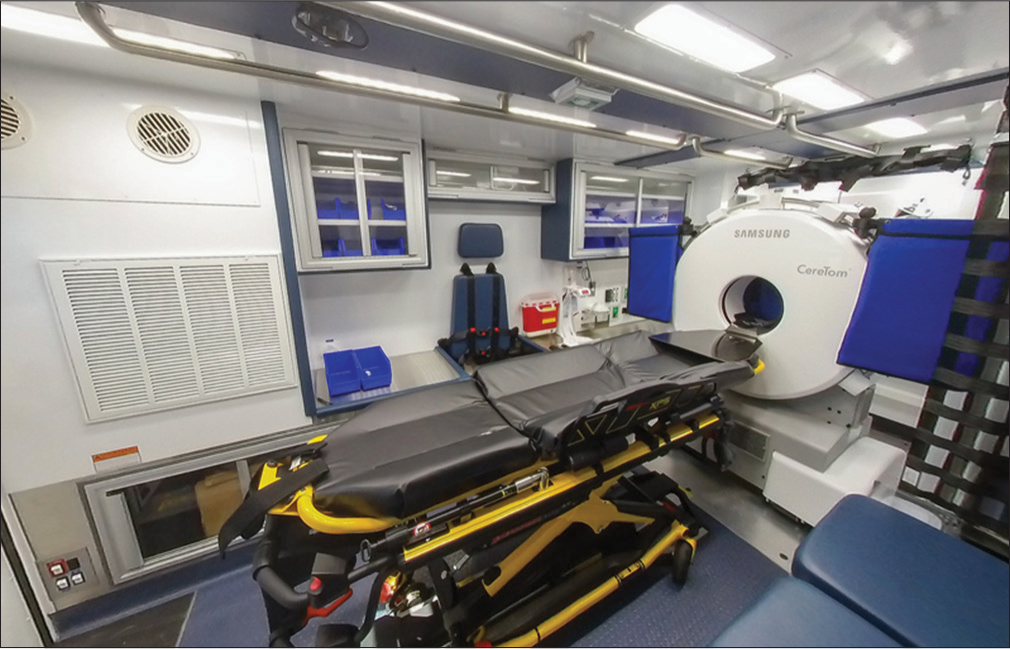
- A standard interior of a mobile stroke unit. Note the presence of standard ambulance equipment, a computed tomography scanner, and point-of-care laboratory equipment. Photo courtesy of Frazer, Ltd.
LOCATIONS
In 2010, the University Hospital of the Saarland published their initial results of the first MSU. They provided a proof of concept, with an average call to decision time of 35 min.[18] The second MSU was created in Berlin and has been clinically used since February 2011.[19] Since these pioneers developed the first MSUs, many others have been created and deployed. The first MSU in the United States was developed in Houston, Texas and has been in clinical use since May 2014.[20] A list of MSUs currently active at the time of this publication can be seen in (Figure 2).[21]

- World map noting the locations of all of the currently functioning mobile stroke units. Note that the first in the world was in Saar, Germany and the first in the United States was in Houston, Texas.
RESULTS
As has been discussed, stroke care is extremely time sensitive, lending itself to the mantra, “time is brain.” Once a patient arrives at a stroke center, care can be streamlined to expedite the delivery of personalized, evidence-based stroke treatment.[15] This, however, does nothing to accelerate pre-hospital care. Intuitively, an MSU is a first step toward fast-tracking patients in this phase. Earlier CT scans, delivery of tPA, proper triage, and on-scene goal-directed care were the primary goals of an early institution of MSUs.[22] It was thought that these early benefits would shorten hospital length of stay and improve long-term outcomes in patients that were treated in MSUs.[22] Since their application, multiple studies have shown both the benefits and limitations of such care.
The first randomized control trial by the University of Saarland group found a decrease of 41 min from stroke alarm to therapeutic decision in the MSU group, 35 min total, compared to the control group, and 76 min total (P < 0.0001).[22] The trial was halted following the interim analysis of the first 100 patients due to the profound positive impact MSUs had on the time to delivery of therapy.[22] Notably, this trial failed to show statistical significance in the improvement of mRS on follow-up, likely due to its short time frame of only 7 days.[22] It did, however, provide concrete “proof of concept” that MSUs could, in fact, be deployed effectively and safely to provide access to therapy in a shorter time period.
This study was followed by the much larger and more in-depth Phantom-S study from the Berlin group.[23] The initial pilot study, published in Neurology, found a decrease in time from stroke alarm to the delivery of therapy when compared to registry times and no adverse outcomes associated with pre-hospital treatment of stroke using IV tPA.[24] This was followed by the large-scale randomized control Phantom-S trial in 6182 patients, which found a decrease of 25 min in time to treatment when the MSU was employed (P < 0.001), an increase in the rate of thrombolysis utilization from 21% in control weeks to 33% when the MSU was employed (P < 0.001), and no change in the rates of intracranial hemorrhage or 7-day mortality.[25] Multiple subsets of data from the study were further analyzed. Ebinger et al. showed that the rate of “golden hour” thrombolysis (treatment delivered within 60 min of symptom onset) increased from 1.1% to 10.1% with use of MSUs and that this was associated with higher rates of discharge to home compared to nursing home (odds ratio [OR] 1.93, P = 0.02).[26] Thus far, studies had only examined short-term outcomes with data on long-term outcomes lacking. In 2016, a sub-study was published in the Lancet showing that for secondary outcomes the 3 months 0–3 mRS (83% in MSU patients, 74% in conventional care, P = 0.004) and 3 months mortality (6% in MSU patients, 10% in conventional care, P = 0.022) were improved in MSU patients.[27] The study’s primary endpoint, disability-free care, trended toward an improvement in MSU patients but failed to reach statistical significance (53% MSU patients, 47% conventional care, P = 0.14).[27]
A retrospective analysis of the results from the Berlin group, published in 2018, attempted to examine the benefits of MSUs in ischemic stroke over a 4 years period (2011–2015).[28] The primary outcome was an mRS of 0–3 at 3 months and secondary outcomes were symptomatic intracranial hemorrhage and 3-month survival status.[29] They showed the use of an MSU was associated with a median reduction of 38 min from symptom onset to treatment compared with conventional care.[28] In addition, starting thrombolytic therapy in the MSU was associated with higher probability of an mRS of 0–3 (OR 1.99; 95% confidence interval 1.02–3.87) but not an improved 3-month survival rate.[28] Importantly, the faster delivery of intravenous thrombolytics was not associated with an increase in the rate of symptomatic intracranial hemorrhage.[28]
Multiple “systems” benefits, with the potential to improve triage and delivery of care to stroke patients, have also been found in studies. In a different subset analysis of the Phantom-S trial, patients with ischemic strokes were more likely to be sent to hospitals without dedicated stroke units in the conventional care group (10.1%) when compared to the MSU group (3.9%, P < 0.01).[29] Similarly, patients with hemorrhagic stroke were delivered to a hospital without a neurosurgery department equipped to deal with hemorrhages in 43.0% of the conventional care group compared to 11.3% in the MSU group (P < 0.01).[29] Finally, a pilot study from Houston, Texas, the first MSU site in the United States, discussed the potential for improving time to endovascular treatment MSU patient, which, given the recent paradigm shifts in invasive stroke treatment, may have a profound effect on outcomes.[30]
CONCLUSION
While these studies all showed promising results, they were not without limitations. Although none showed an increase in adverse events, such as hemorrhagic conversion or mortality, it is difficult to ascertain the degree of bias in these studies. Blinding of the use of MSUs is nearly impossible due to the obvious inherent differences in their treatment. In addition, with the exception of one subset of the Phantom-S study and a newer 2018 study out of Berlin, no other group has shown consistent differences in long-term outcomes with the use of MSUs.[27,28] As a result, no study has done an effective cost-benefit analysis. Another confounding variable is all of these studies compare care delivered by a specialized MSU team and one hospital to conventional care delivered by multiple hospitals. NIHSS is assessed in MSU patients earlier than in patients who receive conventional care, and subsequent worsening or improvement could result in a type of lead-time bias. Finally, the above studies do not delve into the utility of MSUs in an urban compared to a suburban setting, where benefit might not be equivalent. Future studies will target these limitations and ultimately, may show MSU to be cost-effective and provide durable, long-term outcomes benefit.[31]
Declaration of patient consent
Not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Heart disease and stroke statistics-2016 update: A report from the American heart association. Circulation. 2016;133:e38-360.
- [Google Scholar]
- Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581-7.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of alteplase for acute stroke on the distribution of functional outcomes: A pooled analysis of 9 trials. Stroke. 2016;47:2373-9.
- [CrossRef] [PubMed] [Google Scholar]
- Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15:1138-47.
- [CrossRef] [Google Scholar]
- Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285-95.
- [CrossRef] [PubMed] [Google Scholar]
- A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11-20.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019-30.
- [CrossRef] [PubMed] [Google Scholar]
- Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009-18.
- [CrossRef] [PubMed] [Google Scholar]
- Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296-306.
- [CrossRef] [PubMed] [Google Scholar]
- Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695-703.
- [CrossRef] [Google Scholar]
- Treatment with tissue plasminogen activator in the golden hour and the shape of the 4.5-hour time-benefit curve in the national united states get with the guidelines-stroke population. Circulation. 2017;135:128-39.
- [CrossRef] [PubMed] [Google Scholar]
- Public education strategies to increase awareness of stroke warning signs and the need to call 911. J Public Health Manag Pract. 2008;14:e17-22.
- [CrossRef] [PubMed] [Google Scholar]
- Designing a message for public education regarding stroke: Does FAST capture enough stroke? Stroke. 2007;38:2864-8.
- [CrossRef] [PubMed] [Google Scholar]
- Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation. 2011;123:750-8.
- [CrossRef] [PubMed] [Google Scholar]
- Improving door-to-needle times in acute ischemic stroke: the design and rationale for the American Heart Association/American Stroke Association’s Target: Stroke initiative. Stroke. 2011;42:2983-9.
- [CrossRef] [PubMed] [Google Scholar]
- “Mobile stroke unit” for hyperacute stroke treatment. Stroke. 2003;34:e44.
- [CrossRef] [PubMed] [Google Scholar]
- Bringing the hospital to the patient: First treatment of stroke patients at the emergency site. PLoS One. 2010;5:e13758.
- [CrossRef] [PubMed] [Google Scholar]
- Prehospital thrombolysis: a manual from Berlin. J Vis Exp. :e50534.
- [CrossRef] [PubMed] [Google Scholar]
- Establishing the first mobile stroke unit in the United States. Stroke. 2015;46:1384-91.
- [CrossRef] [PubMed] [Google Scholar]
- Personal correspondance between Dr. Grotta to Dr. Bhalla
- Diagnosis and treatment of patients with stroke in a mobile stroke unit versus in hospital: a randomised controlled trial. Lancet Neurol. 2012;11:397-404.
- [CrossRef] [Google Scholar]
- PHANTOM-S: the prehospital acute neurological therapy and optimization of medical care in stroke patients - Study. Int J Stroke. 2012;7:348-53.
- [CrossRef] [PubMed] [Google Scholar]
- Prehospital thrombolysis in acute stroke: Results of the PHANTOM-S pilot study. Neurology. 2013;80:163-8.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of the use of ambulance-based thrombolysis on time to thrombolysis in acute ischemic stroke: A randomized clinical trial. JAMA. 2014;311:1622-31.
- [CrossRef] [PubMed] [Google Scholar]
- Effects of golden hour thrombolysis: a prehospital acute neurological treatment and optimization of medical care in stroke (PHANTOM-S) substudy. JAMA Neurol. 2015;72:25-30.
- [CrossRef] [PubMed] [Google Scholar]
- Functional outcomes of pre-hospital thrombolysis in a mobile stroke treatment unit compared with conventional care: An observational registry study. Lancet Neurol. 2016;15:1035-43.
- [CrossRef] [Google Scholar]
- Effects of prehospital thrombolysis in stroke patients with prestroke dependency. Stroke. 2018;49:646-51.
- [CrossRef] [PubMed] [Google Scholar]
- Improved prehospital triage of patients with stroke in a specialized stroke ambulance: Results of the pre-hospital acute neurological therapy and optimization of medical care in stroke study. Stroke. 2015;46:740-5.
- [CrossRef] [PubMed] [Google Scholar]
- Benefits of stroke treatment using a mobile stroke unit compared with standard management: The BEST-MSU study run-in phase. Stroke. 2015;46:3370-4.
- [CrossRef] [PubMed] [Google Scholar]
- Benefits of stroke treatment delivered using a mobile stroke unit trial. Int J Stroke. 2018;13:321-7.
- [CrossRef] [PubMed] [Google Scholar]






