Translate this page into:
Safety and outcomes with use of FlowTriever for mechanical thrombectomy i n acute pulmonary embolism


*Corresponding author: Samridhi Gulati, Department of Pulmonary and Critical Care Medicine, Corewell Health Grand Rapids Hospitals, Grand Rapids, United States. samridhi9493@gmail.com
Joseph H. Pitcher, Department of Pulmonary and Critical Care Medicine, Corewell Health Grand Rapids Hospitals, Grand Rapids, United States. joseph.pitcher@corewellhealth.org
-
Received: ,
Accepted: ,
How to cite this article: Gulati S, DeJonge J, Shrestha NK, Marsy D, Khan MM, Berjaoui W, et al. Safety and outcomes with use of FlowTriever for mechanical thrombecto my in acute pulmonary embolism. Am J Interv Radiol. 2024;8:17. doi: 10.25259/AJIR_33_2024
Abstract
Objectives:
Mortality in the pulmonary embolism (PE) risk categories has historically been reported between 30% and 40% in high-risk and <15% in intermediate-risk group. In those who survive, there is a high rate of morbidity with dyspnea and exercise intolerance. Advanced therapies with a favorable safety profile have the potential to improve outcomes. We present the largest single-center data set studied to-date for safety, mortality, and outcomes post-mechanical thrombectomy including functional assessment 3 months post-discharge.
Material and Methods:
We analyzed retrospective database of patients with PE undergoing catheter directed mechanical thrombectomy (CDMT). We report clinical characteristics and outcomes stratified by PE risk categories. Comparison in the groups has been made using analysis of variance method.
Results:
A total of 365 patients were evaluated in the CDMT group. Among these 81 (22%) presented with high-risk and 261 (71%) with intermediate-risk PE. The average age at diagnosis was 61 ± 17 years with male-to-female distribution ratio of 1.2. Most common risk factors being reduced mobility (18%), malignancy (15%), recent surgery (13%), and hormonal therapy (12%). Mortality within 30 days of PE diagnosis was 8.6% (7/81) in high-risk, 1.7% (4/230) in intermediate-high-risk groups. There were no deaths in intermediate-low and low-risk group post-CDMT. Before thrombectomy, 349 (95%) patients had right heart strain, 307 (84%) had elevated troponin, and 197 (54%) had elevated B-type natriuretic peptide. Post-procedure echocardiogram at 3 month revealed improvement in the right ventricular (RV) fractional area change (27.53 ± 10.38% to 39.73 ± 8.3%, P < 0.01), tricuspid annular plane systolic excursion (10.9 ± 8.3 mm to 21.81 ± 4.75 mm), and RV systolic pressure (43.96 ± 14.48 mmHg to 28.47 ± 7.88 mmHg, P < 0.01). At 3 months post-thrombectomy, the majority (74%) of the patients fell into non-to-negligible functional limitation.
Conclusion:
We present a descriptive analysis of outcomes including improved mortality, and functional assessment of patients undergoing CDMT.
Keywords
Catheter directed mechanical thrombectomy
Descriptive analysis
Echocardiogram
FlowTriever
Mortality
Pulmonary embolism
INTRODUCTION
Pulmonary embolism (PE) is the third most common cause of mortality due to thromboembolic disease, after ischemic heart disease and ischemic stroke.[1] An estimated 60,000–100,000 Americans die each year due to this disease. Sudden death is first symptom in about 25% of people who have PE.[2,3] Many of those who survive live with serious impact on the quality of their life including pulmonary hypertension, exercise intolerance, and dyspnea.[4,5] Up to 53% of patients report exertional dyspnea after diagnosis of PE. In addition, depending on risk factors, up to 30% will suffer from a recurrent episode, with risk being highest within the first 2 years of diagnosis.[6]
Classifying PE severity helps to predict in-hospital and 30-day mortality. High-risk category includes presence of hemodynamic instability. Intermediate-risk category includes elevated cardiac biomarkers and presence of the right ventricular (RV) dysfunction on echocardiogram or computed tomography pulmonary angiography (CTPA) without hemodynamic instability. Acute RV failure with hemodynamic instability is the leading cause of death in high-risk category. Recently, catheter-based interventions have been used to treat high- and intermediate-risk PE, especially in patients who have contraindications to systemic thrombolysis. Therefore, percutaneous catheter-directed treatment, which was earlier considered as Class IIb recommendation, now comes under class IIa recommendation for management of high-risk PE based on recent 2019 European Respiratory Society guidelines.[7,8] Since 2019 guidelines, there have been additional retrospective studies and prospective trials examining the safety and efficacy of catheter-directed mechanical thrombectomy (CDMT).
We previously published our initial experience with 257 patients who had intermediate- or high-risk PE and underwent mechanical thrombectomy using the FlowTriever regarding procedural success, complications, and post-procedure mean pulmonary artery pressures (MPAP).[9] We now report safety data on an additional 108 patients who have underwent mechanical thrombectomy in all risk categories at our institution since the initial publication and provide 3-month follow-up data on echocardiographic findings and functional assessment of this population.
MATERIAL AND METHODS
FlowTriever System (Inari Medical, Irvine, California, USA) was the first Food and Drug Administration (FDA)-cleared mechanical thrombectomy device approved for the treatment of PE in 2018. The large-bore system combines aspiration and mechanical thrombus extraction to obviate the need for intraluminal thrombolytics and their associated bleeding risk.[10,11]
This is a single-center retrospective study of all patients undergoing CDMT through FlowTriever system during November 2019–March 2023. The study was approved by the Local Institutional Review Board (Corewell Health West Michigan) and a consent waiver was obtained. Our cohort includes those patients who did not have COVID-19 at the time of PE response team (PERT) activation. No patients undergoing CDMT during this time were excluded from the analysis. Summary tables of patient characteristics were presented in terms of mean ± standard deviation (SD) or percent value (n) as appropriate depending upon quantitative or qualitative characteristics. PE risk factors are tabulated, and data are shown by graphs for ease of comparison. The results presented in this analysis include in-hospital outcomes and mortality along with 3-month follow-up data.
Primary endpoint
The primary endpoint of this study is to evaluate all-cause mortality within 30 days of PE diagnosis and major bleeding post-procedure. Post-procedure bleeding was defined and categorized by the International Society on Thrombosis and Hemostasias (ISTH) criterion into major and non-major bleeding. Furthermore, mortality is studied beyond 30 days for the entire duration of study.
Secondary endpoint
Secondary endpoints include changes in the right heart hemodynamics evaluated pre and 3-month post-procedure. Variables collected through echocardiography were RV fractional area change (RVFAC), tricuspid annular plane systolic excursion (TAPSE), right ventricular systolic pressure (RVSP), RV systolic function, and dilation.
Additional secondary endpoints include dyspnea as measured by post-venous thromboembolism (VTE) functional status scale and modified Medical Research Council scale 3-month post-procedure.
Statistical analysis
Summary tables of patient characteristics are presented in terms of mean ± SD for quantitative characteristics and by count and n (%) for qualitative characteristics. Any P < 0.05 was deemed as significant. PE has been risk stratified into high-, intermediate-, and low-risk groups. The intermediate-risk group has been further divided into intermediate-high and intermediate-low groups based on the presence of RV strain and/or an elevated B-type natriuretic peptide (BNP) or troponin. The elevated BNP or troponin was determined within 24 h of PERT activation from the hospital standard result. Mortality, hospital length of stay, RV function, and post-thrombectomy functional status were compared between groups and P-value was obtained. Two groups (for example, intermediate high vs. intermediate low-risk group) were compared using t-test, Chi-squared test or their variations as appropriate, whereas more than two groups (for example, PE categories) were compared using analysis of variance method. The hypotheses of equality of echo parameters were tested using the pairwise tests. P-values for the echo parameters at baseline and later were tested using pairwise t-test or Wilcoxon’s as appropriate.
RESULTS
Patient characteristics
From November 2019 to March 2023, a total of 365 patients with high-, intermediate-, and low-risk PE who underwent CDMT were included in this study. While most patients were in high-risk (81) and intermediate-risk category (261), three patients in low-risk category were included who underwent thrombectomy due to clot burden. Patients were divided into these categories based on initial PERT documentation. There were 20 patients who were unclassified to begin with, due to missing parameters at initial documentation, which were included in this study as they underwent CDMT during this time. The mean age at diagnosis was 61.3 ± 16.8 years. Out of the total patient dataset, 200 were male and 165 were female. The majority (90%) of patients were Caucasians. Risk factors were assessed at presentation and each risk-factor was given one point based on patient characteristics. Finally, the weighted average was calculated for each risk-factor [Table 1].
| Risk factors PE | Absolute number | Risk factors % |
|---|---|---|
| Reduced mobility | 87 | 18 |
| Malignancy (active and past) | 69 | 15 |
| Recent surgery or invasive procedure (<4 weeks) |
64 | 13 |
| Smoking | 58 | 12 |
| Hormonal therapy | 55 | 12 |
| Recent hospitalization ≥3 days (<4 weeks) | 37 | 8 |
| Family history of DVT/PE | 28 | 6 |
| Prolonged travel (≥6 h within 4 weeks) | 27 | 6 |
| Recent trauma (<4 weeks) | 26 | 5 |
| Sepsis | 18 | 4 |
| Indwelling catheter | 4 | 1 |
| Pregnancy | 2 | 0 |
DVT: Deep vein thrombosis, PE: Pulmonary embolism
Primary outcomes
Mortality within 30 days of PERT activation occurred in seven out of 81 patients in high-risk, four out of 230 patients in intermediate-high-risk patients [Figure 1]. No mortality occurred within 30 days in intermediate-low-risk and low-risk group. All-cause mortality during the total study period occurred in two additional patients in the high-risk group and 16 additional patients in the intermediate-risk group. Average time between PERT activation to mortality was 28 ± 40.5 days in high-risk patients, 88.2 ± 85.9 in intermediate-high-risk patients, and 229 ± 171 in intermediate-low-risk patients [Table 2].
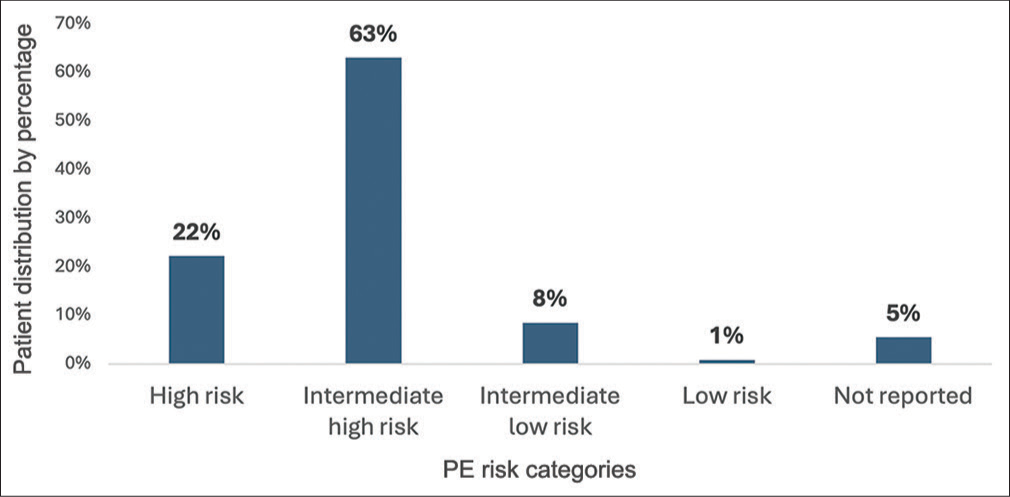
- Patient distribution according to pulmonary embolism risk categories.
| Variable | High (n=81) | Intermediate High (n=230) | Intermediate Low (n=31) | P-value |
|---|---|---|---|---|
| Age at PERT activation | 61.9±16.9 | 62.4±16.1 | 61.1±15.3 | 0.954 |
| Gender=Female, n (%) | 37 (45.7) | 107 (46.5) | 12 (38.7) | 0.715 |
| PERT activation to death (days) | 28±40.5 | 88.2±85.9 | 229±171 | 0.014 |
| Death after PERT activation, n (%) | 9 (11.1) | 17 (7.4) | 3 (9.7) | 0.488 |
| Death within 30 days of PERT activation, n (%) | 7 (8.6) | 4 (1.7) | 0 (0) | 0.018 |
| Hospital length of stay (days) | 5.8±5.8 | 5.1±6.6 | 5.5±6 | 0.687 |
| CTPA -Right heart strain, n (%) | 74 (91.4) | 203 (88.3) | 19 (61.3) | 0.001 |
| BNP+ve/elevated by hospital standard within 24 h, n (%) | 50 (61.7) | 137 (59.6) | 4 (12.9) | <0.001 |
| Troponin+ve/elevated by hospital standard within 24 h, n (%) | 71 (87.7) | 217 (94.3) | 12 (38.7) | <0.001 |
| mMRC score (at 3-month clinic visit) | 0.37±0.76 | 0.39±0.79 | 0.06±0.24 | 0.208 |
| 3-month Post-VTE functional status score, n (%) | 0.413 | |||
| 0 | 26 (32.1) | 69 (30) | 11 (35.5) | |
| 1 | 9 (11.1) | 35 (15.2) | 2 (6.5) | |
| 2 | 9 (11.1) | 19 (8.3) | 5 (16.1) | |
| 3 | 1 (1.2) | 14 (6.1) | 1 (3.2) | |
| 4 | 1 (1.2) | 2 (0.9) | 0 (0) | |
| Not reported | 35 (43.2) | 91 (39.6) | 12 (38.7) |
PERT: Pulmonary embolism response team, CTPA: Computed tomography pulmonary angiogram, BNP: B-type natriuretic peptide, mMRC: Modified medical research council, VTE: Venous thromboembolism
ISTH defined major bleeding occurred in 13 (3.6%) patients and non-major bleeding in 35 (9.6%) patients who were followed for 6 months post-CDMT. Two patients out of the total cohort had recurrent PE during the duration of the study.
PE severity and risk category
Right heart strain was defined as RV/left ventricle (LV) ratio ≥ 0.9 on CTPA or RV/LV diameter ratio ≥ 1 on echocardiography. Right heart strain was present in 95% (349/365) of the patients either on CTPA or echocardiography. Elevation of cardiac biomarkers is associated with worse prognosis and 84% (307/365) patients had elevated troponin and 54% (197/365) had elevated BNP.
Secondary outcomes
RV size and function was evaluated using multiple echocardiographic variables at 24 h within presentation and at a 3-month follow-up clinic visit. Out of 228 patients who had RV size reported on echocardiogram, 185 (81%) patients had normal size with 24 (11%) patients having mild dilation [Figure 2]. Out of 231 patients who had RV systolic function reported on echocardiogram, 193 (83%) patients had normal function, and 22 (11%) patients had mild reduction in the function [Figure 3]. In 99 patients with paired measurement, RVFAC improved from 27.53 ± 10.38% to 39.73 ± 8.3% (P < 0.01), and 76 patients had reduction in RVSP from 43.96 ± 14.48 mmHg to 28.47 ± 7.88 mmHg (P < 0.01). A total of 23 patients with paired measurement of TAPSE had an improvement from 10.9 ± 8.3 mm to 21.81 ± 4.75 mm (P < 0.01) [Figure 4]. However, LV ejection fraction, 24-h post-PE, did not show significant difference in paired measurement from 70 patients (58.6 ± 9.6% to 60.4 ± 9%, P < 0.01).

- Right ventricular size on echocardiogram at 3 months after pulmonary embolism diagnosis. (#:It signifies number of patients in specific color coded right ventricular (RV) size category.)
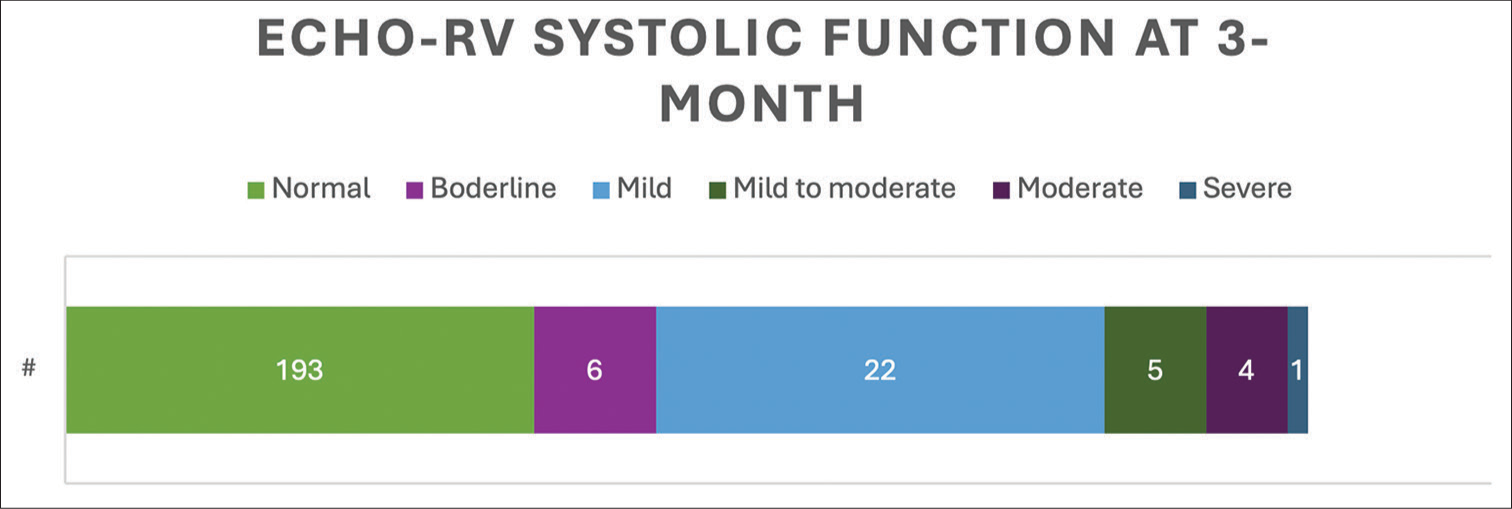
- Right ventricular systolic function on echocardiogram at 3 months after pulmonary embolism diagnosis. (#: It signifies number of patients in specific color coded right ventricular (RV) size category.)

- Paired echocardiographic measurements pre and 3-month post-pulmonary embolism, (a) tricuspid annular plane systolic excursion (10.9 ± 8.3 mm to 21.81 ± 4.75 mm), (b) right ventricular fractional area change (27.53 ± 10.38% to 39.73± 8.3%, P < 0.01), and (c) right ventricular systolic pressure (43.96 ± 14.48 mmHg to 28.47 ± 7.88 mmHg, P < 0.01). (TAPSE: Tricuspid annular plane systolic excursion, RVFAC: Right ventricular fractional area change, RVSP: Right ventricular systolic pressure.)
Dyspnea scores
After thrombectomy, patients were evaluated for dyspnea at a 3-month clinic visit by post-VTE functional status scale.[12] Most patients regardless of PE risk category reported none (106 out of 204; 51.9%) to negligible (46 out of 204; 22.5%) functional limitation (grade O-1 on VTE functional status score). Slight to moderate functional limitations were present in 49 out of 204 (24%) and severe functional limitations were present in 3 out of 204 (1.4%), which included assistance needed in daily activities, pain, or anxiety.
DISCUSSION
Our study is the largest single center study to date of the use of FlowTriever for the management of acute PE. The all-cause mortality in our patients with acute PE undergoing thrombectomy was significantly lower than historical reports.[13-16] Furthermore, procedural effectiveness and safety was demonstrated by a 3.6% risk for major bleeding.
The FlowTriever system is a minimally invasive recent FDA-cleared therapy for treatment of acute PE. This obviates the need for thrombolytic drugs which have been associated with bleeding risks. Many patients could go home shortly after the procedure and remain on anticoagulation during and after recovery. However, per guidelines, CDMT is still recommended only in high-risk PE or in whom thrombolysis is contraindicated or has failed.[17] The FlowTriever pulmonary embolectomy clinical study (FLARE) was the first prospective, single arm, and multicenter investigational device exemption trial published in 2019. In this, CDMT in 104 intermediate-risk patients has proven to be safe and efficacious in terms of improving RV/LV ratio with only 1.9% requiring adjunctive thrombolytic treatment.[18] The FlowTriever Registry for Patients Safety and Hemodynamics (FLASH) registry was subsequently published in 2022 evaluating effectiveness of CDMT in multi-center predominantly intermediate risk PE population.[19] This registry of 250 patients showed improvement in RV size (decreased by 0.36 ± 0.76 [28.3%]) and function (RVSP decreased by 19.1 ± 15.6 mmHg [35.8%]) with a low rate of major bleeding (1.2%). Our study has also shown improvement in echocardiographic parameters including RV function, with 83% having normal function at 3 months, reaffirming the findings from FLARE and FLASH studies.
In 2023, the FlowTriever for acute massive PE study provided data on high-risk population undergoing CDMT.[20] This trial showed a significant reduction in-hospital mortality in FlowTriever subgroup (1/53 [1.9%]) as compared to usual treatment arm (18/61 [29.5%]). However, this study had a total of 53 patients in FlowTriever arm.
Previous reports have suggested persistent RV dysfunction, even 6 months post-PE. A study in submassive PE showed 6-month follow-up echocardiogram, 75% patients had a normal right ventricle, whereas 25% had an abnormal right ventricle.[21] Another study compared systemic thrombolysis with heparin in sub-massive PE. It showed that improved from 11.9 ± 1.4 mm to 21.2 ± 2.3 mm in thrombolysis group versus 12.3 ± 2.1 mm to 19.2 ± 1.2 mm in heparin group at 3 months. This showed an improvement of 78% versus 56% from baseline, respectively.[22] Our study shows 100% increase in TAPSE post-CDMT, demonstrated by 3-month echocardiogram, without the significant bleeding complications as seen with thrombolysis. Our recently published report on effectiveness and safety of large-bore aspiration thrombectomy for intermediate- or high-risk PE has demonstrated a decrease in MPAP from a mean of 32 mmHg before to 24 mmHg after thrombectomy (mean decrease 8 mmHg ± 6 [SD]; P < 0.001).
Patients in our study also experienced long-term improvement in dyspnea as evaluated by post-PE functional assessment score at 3 months, with 74.5% reporting none to negligible physical impairment. In the evaluation of long-term outcomes after PE (ELOPE) study, almost half of the patients with PE (40 of 86) had exercise limitation at 1 year that adversely influenced dyspnea, walking distance, and quality of life. The ELOPE study reported that baseline or residual clot burden was not associated with the outcome. However, in this study, most of the patients were managed only with anticoagulation.[23] Another study showed that patients with PE had substantially higher prevalence of both exertional dyspnea (53.0% vs. 17.3%, odds ratio [OR]: 5.40, 95% confidence intervals [CI]: 4.61–6.32) and wake-up dyspnea (12.0% vs. 1.7%, OR: 7.7, 95% CI: 5.28–11.23) compared to control subjects.[24] More studies are needed to determine the effects of CDMT on long-term functional outcomes after acute PE.
CONCLUSION
Despite advances in CDMT, AC alone remains the treatment of choice for PE among all risk categories. At present, CDMT is recommended in patients with high-risk PE, in whom thrombolysis is contraindicated or failed. In this retrospective study, we report a favorable safety profile and impact on outcomes with use of CDMT in all risk categories. Further randomized controlled trials are needed to compare CDMT versus other treatment modalities in acute PE.
Ethical approval
The study was approved by the Local Institutional Review Board (Corewell Health West Michigan) and a consent waiver was obtained. Date of Approval: March 13, 2023, IRB#: 2023-050. IRB had made the following determination:
WAIVER OF HIPAA AUTHORIZATION: A waiver of HIPAA authorization has been approved per 45 CFR 164.512(i)(2)(ii).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
Dr Michael Knox and Dr Trevor Cummings are currently consultant for Inari Medical Inc.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Global burden of thrombosis: Epidemiologic aspects. Circ Res. 2016;118:1340-7.
- [CrossRef] [PubMed] [Google Scholar]
- Venous thromboembolism (blood clots): Data and statistics. Available from: https://www.cdc.gov/ncbddd/dvt/data.html [Last accessed on 2024 Jul 05]
- [Google Scholar]
- Venous thromboembolism: A public health concern. Am J Prev Med. 2010;38:S495-501.
- [CrossRef] [PubMed] [Google Scholar]
- Health-related quality of life after pulmonary embolism: A cross-sectional study. BMJ Open. 2016;6:e013086.
- [CrossRef] [PubMed] [Google Scholar]
- Persistent right ventricular dysfunction, functional capacity limitation, exercise intolerance, and quality of life impairment following pulmonary embolism: Systematic review with meta-analysis. Vasc Med. 2017;22:37-43.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of recurrence after deep vein thrombosis and pulmonary embolism: A population-based cohort study. Arch Intern Med. 2000;160:761-8.
- [CrossRef] [PubMed] [Google Scholar]
- The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC) 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC) Eur Respir J. 2019;54:1901647.
- [CrossRef] [PubMed] [Google Scholar]
- Catheter-directed treatment of pulmonary embolism: A systematic review and meta-analysis of modern literature. Clin Appl Thromb Hemost. 2017;23:821-9.
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness and safety of large-bore aspiration thrombectomy for intermediate-or high-risk pulmonary embolism. J Vasc Interv Radiol. 2024;35:563-75.
- [CrossRef] [PubMed] [Google Scholar]
- Interventional therapies for acute pulmonary embolism: Current status and principles for the development of novel evidence: A scientific statement from the American heart association. Circulation. 2019;140:e774-801.
- [CrossRef] [Google Scholar]
- Safety and efficacy of acute pulmonary embolism treated via large-bore aspiration mechanical thrombectomy using the inari flowtriever device. J Vasc Interv Radiol. 2019;30:1370-5.
- [CrossRef] [PubMed] [Google Scholar]
- Measuring functional limitations after venous thromboembolism: A call to action. Thromb Res. 2019;178:59-62.
- [CrossRef] [PubMed] [Google Scholar]
- Incidence of mortality and complications in high-risk pulmonary embolism: A systematic review and meta-analysis. J Soc Cardiovasc Angiogr Interv. 2023;2:100548.
- [CrossRef] [PubMed] [Google Scholar]
- Contemporary national trends and outcomes of pulmonary embolism in the United States. Am J Cardiol. 2022;176:132-8.
- [CrossRef] [PubMed] [Google Scholar]
- Acute pulmonary embolism: Mortality prediction by the 2014 European Society of Cardiology risk stratification model. Eur Respir J. 2016;48:780-6.
- [CrossRef] [PubMed] [Google Scholar]
- Antithrombotic therapy for VTE disease: Second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545-608. Erratum in: Chest 2022;162:269
- [CrossRef] [PubMed] [Google Scholar]
- A prospective, single-arm, multicenter trial of catheter-directed mechanical thrombectomy for intermediate-risk acute pulmonary embolism: The FLARE study. JACC Cardiovasc Interv. 2019;12:859-69.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous mechanical thrombectomy in a real-world pulmonary embolism population: Interim results of the FLASH registry. Catheter Cardiovasc Interv. 2022;99:1345-55.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes in high-risk pulmonary embolism patients undergoing flowtriever mechanical thrombectomy or other contemporary therapies: Results from the FLAME study. Circ Cardiovasc Interv. 2023;16:e013406.
- [CrossRef] [PubMed] [Google Scholar]
- Echocardiographic and functional cardiopulmonary problems 6 months after first-time pulmonary embolism in previously healthy patients. Eur Heart J. 2007;28:2517-24.
- [CrossRef] [PubMed] [Google Scholar]
- Six-month echocardiographic study in patients with submassive pulmonary embolism and right ventricle dysfunction: Comparison of thrombolysis with heparin. Am J Med Sci. 2011;341:33-9.
- [CrossRef] [PubMed] [Google Scholar]
- Functional and exercise limitations after a first episode of pulmonary embolism: Results of the ELOPE prospective cohort study. Chest. 2017;151:1058-68.
- [CrossRef] [PubMed] [Google Scholar]
- Dyspnea after pulmonary embolism: A nationwide population-based case-control study. Pulm Circ. 2021;11:20458940211046831.
- [CrossRef] [PubMed] [Google Scholar]







