Translate this page into:
Image-guided Percutaneous Sclerotherapy of Vascular Malformations of the Male Genitalia - A Retrospective Study

-
Received: ,
Accepted: ,
How to cite this article: Brill R, Guntau M, Stangl F, Teusch V, Schramm D, Stoevesandt D, Sadick M, Wildgruber M, Wohlgemuth W. Image-guided Percutaneous Sclerotherapy of Vascular Malformations of the Male Genitalia - A Retrospective Study. Am J Interv Radiol 2019; 3(3):1-11.
Abstract
Purpose:
Regarding genital lesions, the incidence of male external genitalia vascular anomalies is circa 3%, thereof one-tenth tumors and nine-tenth malformations according to the International Society for the Study of Vascular Anomalies classification. Image-guided percutaneous sclerotherapy in male external genitalia vascular malformations has rarely been described. Therefore, a retrospective analysis of sclerotherapy in a series of eight patients was conducted.
Materials and Methods:
The study was IRB approved. Two radiologists reviewed angiographic reports and analyzed interventionally treated male patients with external genitalia vascular malformations between February 2, 2014, and November 11, 2017, at an interdisciplinary tertiary care Vascular Anomalies Center. Inclusion criteria were a slow-flow malformation of the male external genitalia and no interventional treatment before. Operations longer than 1-year past were no exclusion criteria. Patients suffered from lymphatic and/or venous malformations and received percutaneous sclerotherapy. Malformations were treated with polidocanol, ethanol in gel form or OK-432. Patients answered a questionnaire regarding symptoms with repeat after follow-up. The initial state and post-treatment results were compared. Magnetic resonance imaging pre- and post-intervention was assessed. Complications were reported standardized.
Results:
Eight patients with a mean age of 21.6 years suffered from genital swelling, bleeding, thrombophlebitis, lymphorrhea, skin changes, pain, and functional genitourinary symptoms and were treated with sclerotherapy. All patients reported clinical improvement of symptoms during the average follow-up period of 30 months. No complications ensued.
Conclusion:
Sclerotherapy seems to be a safe and effective treatment of slow-flow malformations of the male external genitalia. Due to the low incidence of the disease, multicenter studies are necessary to assess a larger number of cases.
Keywords
Interventional treatment
Male external genitalia
Sclerotherapy
Vascular malformation
INTRODUCTION
Vascular malformations of the male external genitalia represent a rare disease mostly diagnosed in childhood or young adulthood. For adequate therapy, vascular malformations are generally categorized into biological and histological subtypes. In 1982, Mulliken and Glowacki developed a system subclassifying vascular anomalies into hemangiomas and vascular malformations. In 2014, the International Society for the Study of Vascular Anomalies (ISSVA) updated and expanded this classification.[1] Vascular malformations are inborn and may increase proportionally with growth.[2] They consist of abnormal, deformed vascular channels and can affect the arterial, venous, capillary, lymphatic system,[2] or combinations thereof. Due to their hemodynamic properties, vascular malformations are classified into high-flow (arterial and arteriovenous) and low-flow (venous, lymphatic, and capillary) malformations.[3]
As developmental defects, vascular malformations can affect every part of the body,[4] including the external genitalia[5,6] and may present with a wide range of clinical characteristics. Symptoms can be various and include skin discoloration, local swelling and disfigurement, bleeding, thrombophlebitis, lymphorrhea and pain, depending on the affected vascular components, location, and size. As long as vascular malformations are not or only minimally symptomatic, treatment may not be warranted. Indications for treatment are symptomatic or esthetically disturbing lesions.
Psychologically external genitalia vascular malformations can lead to a negative self-image; the physiological function can be profoundly affected.[5,6] Restriction of the health-related quality of life may be another indication for treatment. Treatment options include conservative approaches, interventional treatment, open surgical repair, or any combination thereof.
All patients who received percutaneous sclerotherapy of vascular malformations of the male external genitalia at one tertiary care interdisciplinary Vascular Anomalies Center (VAC) over the past 3 years and 10 months were retrospectively reviewed.
Evaluation included symptoms leading to treatment, different treatment modalities, and treatment courses leading to a satisfactory clinical outcome as well as an improvement in health-related quality of life.
MATERIALS AND METHODS
Study population
All male patients receiving image-guided percutaneous sclerotherapy of the external genitalia for vascular malformation during the review period from February 2, 2014, to November 11, 2017, at a tertiary care VAC were analyzed retrospectively. Analyses included angiography reports as well as detailed review of patients’ records. IRB approval was obtained before study begin. During the review period, eight male patients received one or more sclerotherapy treatments for symptomatic vascular malformations of the male external genitalia. Age range at the time of first treatment was 5–51 years with a mean age of 21.6 years. 50% were children (aged 18 or less) at first treatment, 50% were adults. For an accurate diagnosis, magnet resonance imaging (MRI) was performed with a focus on the genitalia before and after treatment to evaluate the malformations’ extension and diagnose the exact vascular malformation type according to the ISSVA classification. Inclusion criteria were any kind of slow-flow malformation of the male external genitalia according to the ISSVA classification, simple lymphatic or venous or combined type of malformation, singular appearance, or together with affection of other body regions, associated with other anomalies or not, and no interventional treatment at the genitalia before starting image-guided percutaneous sclerotherapy in our VAC. Operations longer than 1-year past were no exclusion criteria, recently performed surgery was exclusion criteria. Vascular malformation with high-flow, arterial involvement, laser therapy of this body region, and general drug therapy of the vascular malformation were exclusion criteria.
Treatment population was structured in three subgroups referring to ISSVA. Of the 8 patients reviewed two patients had a simple venous malformation (VM). The particular characteristics on MRI are a hypointense signal in T1- and a hyperintense signal in T2-weighted sequences, dynamic slow-flow characteristics in time-resolved imaging and enhancement after contrast medium application in T1. If there is a communication to deep draining venous system seen in MRI, ethanol in gel form was applied. Due to its high viscosity, this sclerosant remains in situ. VM without communication to the deep draining venous system was treated with Polidocanol to achieve a good distribution due to its less viscosity. Referring to these characteristics, the two patients with simple VM received polidocanol. The second subgroup is represented by four patients with simple lymphatic malformation (LM) showing hypointense signal in T1- and a hyperintense signal in T2-weighted sequences without dynamic slow-flow characteristics in time-resolved imaging and no (or exclusively cyst wall) enhancement after contrast medium application in T1. This distribution pattern warranted the use of OK-432 in this subgroup as a sclerosant for LM. The last subgroup contains two patients with combined capillary veno LM (CVLM) malformation. The capillary component is a visual diagnosis, the VM and LM component is diagnosed in MRI as described above. In both patients, the leading component of the CVLM was the VM, once with communication to the deep draining venous system (usage of ethanol in gel form), and once without communication (usage of polidocanol). Percutaneous sclerotherapy was performed with ultrasound guidance under fluoroscopy and digital subtraction angiography.
Data collection included patient history, physical examination, as well as follow-up with a questionnaire, physical examination, and MRI.
Patient history and treatment indications were discussed in a multidisciplinary conference together with the patient’s urologist before treatment was administered. Indications for treatment were defined in four main categories: Pain (alguria and swelling/compression pain), infection (erysipelas and caused by lymphorrhea), functional disorders (dysuria), and disfiguration. Symptoms and clinical success were recorded at each consultation following sclerotherapy. Follow-up after interventional treatment was scheduled 2–9 months after each intervention. MRI before and after treatments were compared and evaluated (Figures 1 and 2). A routinely applied questionnaire assessed lymphorrhea and skin involvement before and after treatments and categorized into high grade (+++), intermediate (++), low grade (+), and asymptomatic (−). The pain was routinely scored with a VAS pain scale. Only patients with complete documentation were included.
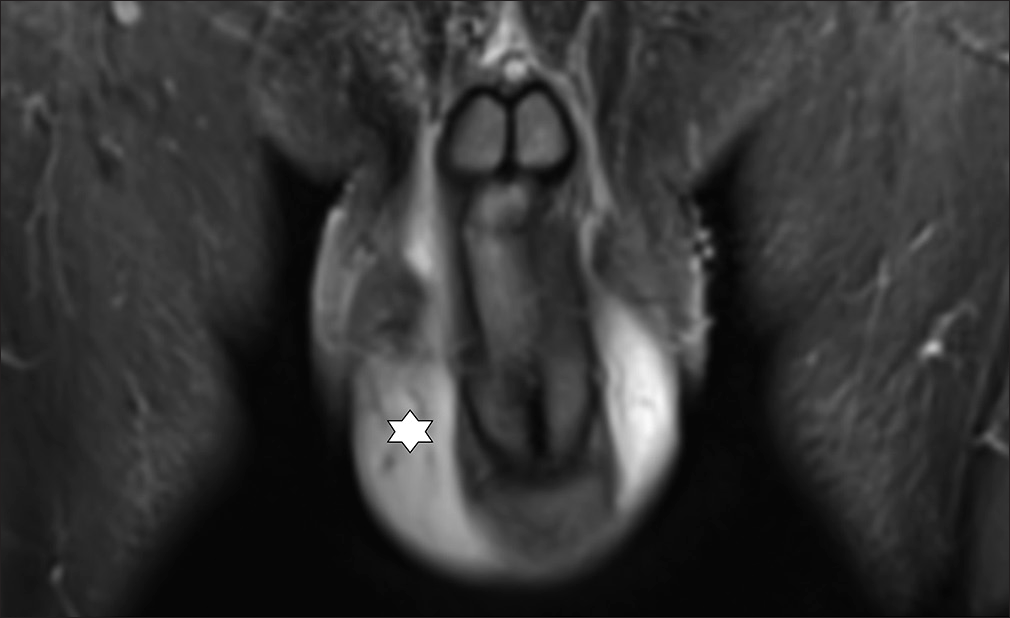
- 51 year old male with LM scrotum and penis. Coronar MRI-image (STIR) shows swelling and oedema on scrotum and penis (star). Before Treatment.

- 51 year old male with LM scrotum and penis. Coronar MRI-image (STIR) shows swelling and oedema on scrotum and penis (star). After Treatment.
Interventional treatment, sclerosants, and procedural steps
Evaluated steps were the number of interventions, technique, punctured vessels, number of punctures during each intervention, as well as the material used for each treatment. Conscious sedation or general anesthesia was documented.
Initially, an ultrasound examination was performed, and the malformation was punctured with a 23-gauge needle (Sterican®, B. Braun, Melsungen, Hesse, Germany) under ultrasound guidance. This was followed by aspiration and a varicography or lymphangiography under fluoroscopy with iodinated contrast medium (Imeron 300®, Bracco, Milan, Lombardy, Italy) to verify the correct needle position and to identify the volume of the punctured malformation compartment. A connection to the deep venous system was excluded. In LM, a lymphangiography to detect lymphatic anomalies was performed before treatment. Afterward, a sclerosant was injected in one or several punctures, followed by fluoroscopy to control the distribution (Figures 3 and 4).

- 6 year old male with CLOVES-Syndrome. CVLM of scrotum and penis. Procedural image of intervention in angiography (arrow).

- 6 year old male with CLOVES-Syndrome. CVLM of scrotum and penis. Visualisation of procedural image of intervention in angiography (arrow).
Three different sclerosing agents were used according to the indication (Table 1). Polidocanol (Aethoxysklerol®, Kreussler & Co. GmbH, Wiesbaden, Hesse, Germany) a detergent sclerosant containing a small amount of ethanol, ethanol in gel form (ScleroGel®, ab medica Deutschland GmbH & Co. KG Düsseldorf, North Rhine-Westphalia) consisting of jellified, highly viscous ethanol, and OK-432 (Picibanil®, Chugai Pharmaceutical Co., Ltd., Roche group, Tokyo, prefecture Tokyo, Japan). OK-432 was injected into patients with LMs. This sclerosant is an immune stimulant consisting of lyophilized bacterial cultures (streptococcal strains; Group A type III 3) which are treated with benzylpenicillin and hydrogen peroxide.[7] The sclerosant triggers an immune reaction causing tissue contraction and vessel sclerosis in LMs.[8]
| Name | Shortcut/brand | Indication | Special feature |
|---|---|---|---|
| OK-432 | Picibanil® | LM | |
| Polidocanol | Aethoxysklerol® | VM | Without communication to draining venous system less viscosity |
| Polidocanol air foamed Ethanol in gel form |
ScleroGel® | VM | With communication to draining venous system high viscosity |
Pure or jellified ethanol as a gel can be used to generate a similar effect of vessel wall destruction through a different mode of action. Pure ethanol solution contains 96% of alcohol in liquid condition. It denatures blood proteins, destructs the endothelial cells and breaks down the vascular wall.[9,10] It is widely used in this indication because it is cheap, easily available, and effective and has a low recurrence rate.[11,12] Ethanol in gel form is a highly viscous gel based on pure ethanol and cellulose. Due to its viscous nature, it remains in longer contact to the vessel wall and causes accelerated dehydration and sclerosing of the vessel. This viscous agent has a reduced risk of propagation to the systemic circulation, the local sclerosing effect is high, and the complication rate low.[13,14]
The chemical detergent aethoxysclerol (Polidocanol) was used in the highest concentration (3%) and administered as a foam (1:4 air-foamed using the EasyFoam Kit®, Kreussler & Co. GmbH, Wiesbaden, Hesse, Germany) to further increase the sclerosing effect.
After treatment a local 24 h compression bandage (Peha-haft® 8 cm × 20 m, Hartmann, Heidenheim, Baden-Wuerttemberg, Germany) was administered, local cooling applied, and low-molecular-weight heparin (Clexane®, Sanofi-Aventis, Frankfurt/Main, Hesse, Germany) at prophylactic dose was prescribed for 7 days in VM to prevent thrombosis. Each patient was presented to a urologist post-intervention. A repetition of the interventional treatment was performed if the main symptom led to treat (pain, function, infection, and disfiguration) did not obviously improve. The interval for repeat-interventions was not performed earlier than 3 months after prior intervention. Any procedural or post-procedural complication was documented through Clavien-Dindo Classification.[15] To report possible complications, patients were visited after intervention and each day of the inpatient stay (1–2 days after the day of intervention) by the performing radiological interventionalist and a urologist. Outpatient follow-up consultation after each treatment was scheduled within 2–9 months. This included an interview and questionnaire about symptom improvement and visual inspection (skin and lymphorrhea) (Figures 5 and 6), pain scale (VAS of pain) and assessment of relief by the interventionalist.

- 6 year old male with CLOVES-Syndrome. CVLM of scrotum and penis. Clinical picture before treatment (arrow).

- 6 year old male with CLOVES-Syndrome. CVLM of scrotum and penis. Clinical picture after treatment (arrow).
RESULTS
Affected structures of the male external genitalia included scrotum and penis. 62.5% had involvement of both, penis and scrotum, in 25% only the scrotum was involved, in 12.5% only the penis.
According to the ISSVA classification, all patients suffered from low-flow malformations; 50% of the patients had a LM, 25% a VM, and 25% a combined CVLM (Table 2). In five patients, pathological changes of the external genitalia were part of malformation syndromes (“vascular anomalies associated with other anomalies”): Two patients had a proven CLOVES syndrome (acronym for congenital, lipomatous, overgrowth, vascular malformations, epidermal naevi, and Spinal/Skeletal anomalies or scoliosis) with mutation in the PIK3CA gene (Table 3). One patient had a central conducting lymphatic anomaly (CCLA), verified with lymphangiography, one patient suffered from a Klippel-Trenaunay Syndrome, and one patient had an unclassified syndrome combined with preterm birth associated with macrocephalus, mitral valve insufficiency, hydrocele testis, and mental retardation.
| Simple malformations | Combined malformations |
|---|---|
| Capillary malformation (C) | CVM, CLM |
| Lymphatic malformation (L) | LVM, CLVM |
| Venous malformation (V) | CAVM |
| Arteriovenous malformation | CLAVM |
| Arteriovenous fistula | CLAVM |
ISSVA: International Society for the Study of Vascular Anomalies, CVM: Congenital vascular malformations
| Pat. | Pathology/syndrome (s) | Type of genital vascular malformation | Age | Number of treatments | Laterality of treatments | Amount of sclerosant in total | Prior treatments |
|---|---|---|---|---|---|---|---|
| 1 | CCLA (s) | LM Scrotum, Penis | 25 | 4 | Right | 3.2 KE OK–432 | Goin exploration excision of lymph vesicles |
| 2 | Unknown syndrome (s) | LM Scrotum | 30 | 1 | Right 0.5 | KE OK-432 | Hydrocele operation |
| 3 | Singular lymphedema | LM Scrotum, Penis | 51 | 1 | Bilateral | 1 KE OK-432 | none |
| 4 | Singular lymphedema | LM Scrotum, Penis | 43 | 3 | Right | 3.5 KE OK-432 | none |
| 5 | CLOVES (s) | CVLM Scrotum, Penis | 6 | 1 | Bilateral | 1.2 ml Polidocanol | none |
| 6 | CLOVES (s) | CVLM Scrotum | 5 | 1 | Bilateral | 1,5 ml Ethanol-gel | orchipexy |
| 7 | Klippel-trenaunay syndrome (s) | VM scrotum, penis | 5 | 1 | Bilateral | 1 ml Polidocanol | none |
| 8 | VM left lower quadrant | VM penis | 8 | 1 | Left | 1.5 ml Polidocanol | none |
VM: Venous malformation, LM: Lymphatic malformation, CCLA: Central conducting lymphatic anomaly
Three patients were not syndromatic. Two of them suffered from a localized LM of the external genitalia and one patient from a VM of the left lower quadrant including the external genitalia.
Six patients received a single treatment and two patients several interventional treatments (one patient had three and one four interventions).
Overall 13 sclerotherapy procedures were performed in this patient collective.
Three patients had a surgical procedure in the past before interventional treatment, but none had recently performed surgery: One patient had a surgical procedure concerning only the external genitalia in terms of an orchidopexy 4 years before the beginning of the review period. One patient had multiple genital and inguinal operations: A resection of a hydrocele, a circumcision and inguinal hernia repairs twice, and last surgical treatment 12 years ago. Moreover, the third patient had an inguinal operation with groin exploration and excision of lymphatic vesicles 9 years ago.
The two patients with CLOVES syndrome had interventional treatments of vascular malformations in other parts of the body in the past: One patient had several sclerotherapy treatments of a VM of the leg and a coiling of the superior mesenteric vein 3 years before staring sclerotherapy, the other patient had a vascular plug in VM of the leg to prevent thrombembolic events 2 years before.
The most frequent symptom was swelling with an incidence of 100%. 62.5% of the patients suffered from lymphorrhea and skin involvement, lymphangioma circumscriptum, and erysipelas were present in 37.5% each, bleeding in 25%, and thrombophlebitis in 12.5%. Relevant pain was present in only three patients. 75% of patients had a combination of symptoms, whereas 25% suffered from swelling only. Specific genitourinary symptoms were found in four patients: Two patients with dysuria, one with alguria and one with hematuria (Table 4). No bladder outlet obstruction, erectile dysfunction or dyspareunia were reported. Main symptom for percutaneous sclerotherapy was a pain in 37.5%, function and infection in 25% each, and disfiguration in 12.5%. All of the patients had a bilateral clinical involvement and also complained of bilateral symptoms. In 50% of patients, the intervention was performed bilaterally; in 50% the more severely affected side was treated. Before treatment sessions, patients with lymphedema were especially examined with an intranodal lymphangiography to verify or exclude a problem in the central conducting lymphatic system (Figures 7-13). Of four patients with LM, only one CCLA was detected.
| Patient | Swelling (yes/no) | Lymphorrhoe (yes/no) | Skin involvement (Erysipel (E), Lymphangioma circumscriptum (L), Bleeding (B), Thrombophlebitis (T) |
Pain (yes/no) | Specific genitourinary symptoms | Main symptom for treatment |
|---|---|---|---|---|---|---|
| 1 | Y | Y | L | N | Dysuria | Function |
| 2 | Y | Y | E | N | Infection | |
| 3 | Y | Y | L, E | N | Infection | |
| 4 | Y | N | N | N | Disfiguration | |
| 5 | Y | N | N | Y | Pain | |
| 6 | Y | N | N | N | Dysuria | Function |
| 7 | Y | N | B, T | Y | Alguria | pain |
| 8 | Y | N | B | Y | Hematuria | pain |

- 43 year old male with singular lymphedema, LM scrotum and penis. Procedural image of intervention in angiography (arrow). Intranodal lymphangiography left inguinal region.

- 43 year old male with singular lymphedema, LM scrotum and penis. Procedural image of intervention in angiography (arrow). Intranodal lymphangiography right inguinal region.
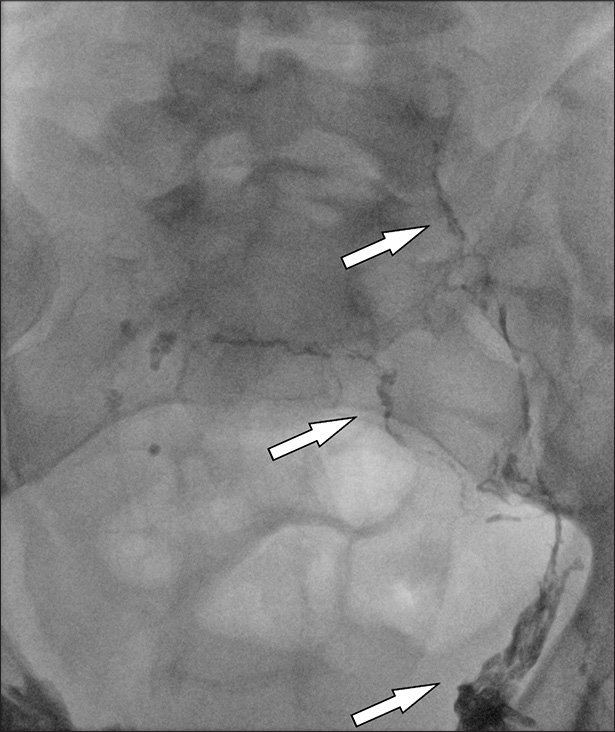
- 43 year old male with singular lymphedema, LM scrotum and penis. Procedural image of intervention in angiography (arrows). Intranodal lymphangiography pelvic lymphatic drainage.

- 43 year old male with singular lymphedema, LM scrotum and penis. Procedural image of intervention in angiography (arrows). Intranodal lymphangiography pelvic lymphatic Drainage, follow up for progress lymphatic drainage.

- 43 year old male with singular lymphedema, LM scrotum and penis. Overview image of intranodal lymphangiography. Procedural image of intervention.

- 43 year old male with singular lymphedema, LM scrotum and penis. Procedural image of intervention in angiography. Intranodal lymphangiography lymphatic drainage thoracial, venous angle left (arrow).

- 43 year old male with singular lymphedema, LM scrotum and penis. Procedural image of intervention in angiography. Enlarged image of intranodal lymphangiography lymphatic thoracic drainage, left venous angle (arrow).
A direct puncture of the main component of the lymphatic or venous vessel was performed ultrasound-guided. Regarding the first treatments of all patients 2.8 punctures were needed averagely. A singular puncture was sufficient in 33%. The maximum number of punctures to achieve a favorable result was 6 times.
The treatment was considered complete when optimal dissemination of the sclerosant was seen in fluoroscopy. OK-432 was used in 50%, polidocanol in 37.5%, and ethanol in gel form in 12.5% of patients. The choice of sclerosants depended on the type of vascular malformation according to the ISSVA. All LM were treated with OK-432, as it is known to be safe and effective in lymphatic vessels by an immune reaction of the vessel wall. We did not treat anyone with another lymphatic sclerosants such as doxycycline or bleomycin because we have the most experience with OK-432. To treat VM, we chose polidocanol or ethanol in gel form because it is both effective and causes less side effects than pure ethanol. If communication to the deep draining venous system was seen in MRI, ethanol in gel form was applied due to its high viscosity; if not, polidocanol was used. Each patient was treated with the same type of sclerosant regardless of the frequency of treatments.
OK-432 was used with a total dose between 0.2 KE and 1.5 KE, averagely 0.9 KE per procedure (1 KE translates to 0.1 mg streptococcal lysate); the maximal dose recommended is 2 KE/injection.[16] The dosage of polidocanol was averagely 1.2 ml (min. 1.0 ml, max. 1.5 ml 3% solution, and 1:4 air foam), the dosage of ethanol in gel form was 1.5 ml (once used).
Repeat procedures were necessary for 25% of the patients treated with OK-432, the patients with other sclerosants needed no repetition. Treatment of patients with several interventions was completed when symptoms were reduced to the best possible minimum, in this patient collective within 3 years. Regarding the subgroups of main symptoms, pain (3 patients) was reduced >70% on average on VAS. Full function (no dysuria) was received in the two patients with functional problems. The two patients who were treated due to infection had no further periods of infection. Disfiguration subjectively ameliorated in the one patient who suffered mainly from disfiguration. To summarize, the main symptomled to treat disappeared in 50%, in 50% it improved obviously. The leading unspecific symptom – swelling – ameliorated in all patients (assessment of lesions’ extent in MRI showed a reduction of the lesion size), but disappeared in only two patients completely (Table 5). All patients reported clinical improvement, one patient even a complete resolution of symptoms. The mean follow-up period between first treatment and last consultation was 30 months (7–40 months). Follow-up included an interview and questionnaire about symptom improvement and visual inspection (skin and lymphorrhea) (Figures 5 and 6), pain scale (VAS of pain), and assessment of relief by the interventionalist and MRI. All patients with polidocanol were in need of only one sclerotherapy treatment and had first follow-up on average after 5 months.[3,4,9] The patient who received ethanol in gel form had a first follow-up after 7 months. Then, follow-up of these four patients was yearly, as they were children with extensive malformations of other body regions (three with syndromes and one with quadrant VM). To summarize, two (1 patient) to three (3 patients) follow-ups were done in this collective until the end of the review period. The subgroup of patients treated with OK-432 had follow-up more frequently due to repetitional sclerotherapy sessions in 50% of these patients (on average 3.25 follow-ups in the review period). After sufficient relief of main symptom leading to treat, one more follow-up was done. No procedural and post-procedural complications occurred referring to Clavien-Dindo classification.
| Patient | Swelling in MRI before/after | Lymphorrhea before/after | Bleeding | Skin involvement before/after | Pain before/after (VAS) | Relief no/partial/total |
|---|---|---|---|---|---|---|
| 1 | +++/+ | +++/+ | -/- | ++/+ | 0/0 | Partial |
| 2 | ++/- | ++/- | -/- | +/- | 0/0 | Total |
| 3 | +++/+ | +++/- | -/- | +++/+ | 0/0 | Partial |
| 4 | ++/+ | -/- | -/- | -/- | 0/0 | Partial |
| 5 | +++/+ | -/- | -/- | -/- | 7/2 | Partial |
| 6 | +++/+ | -/- | -/- | -/- | 0/0 | Partial |
| 7 | ++/+ | -/- | ++/- | +++/- | 8/1 | Partial |
| 8 | +/- | -/- | ++/- | ++/- | 5/2 | Partial |
VAS: Vascular Anomalies Center, MRI: Magnetic resonance imaging
DISCUSSION
The aim of this study was the collection of descriptive data of male patients with external genital vascular malformations undergoing interventional treatment to warrant further studies. This disorder is exceedingly rare and systematic reports concerning this special patient collective are almost non-existent. In an American report, 2.7% of all male patients with genital lesions had a vascular malformation. No data about treatment modalities were provided.[6]
An interdisciplinary approach is strictly important to choose the appropriate therapeutic modality, interventional, surgical, or pharmacological treatment in each particular case.[17] For this reason, a urologist was part of the attending doctors in this study, and if pharmacological treatment seemed to be more expedient, interdisciplinary care was expanded.
As sclerotherapy is currently known as the primary treatment modality of LM and VM,[18] this study focused on image-guided percutaneous sclerotherapy of LMs and VMs of the external genitalia. Literature regarding sclerotherapy of vascular malformations mainly focuses on the cervicofacial region, extremities, and trunk.[19]
Sclerotherapy of the male external genitalia is associated with specific challenges: Technically, it is a peripheral draining area, and for the avoidance of necrosis, sclerosing agents must be dosed carefully and administered under fluoroscopic guidance. Apart from the technical problems, it is challenging to lead the patient through this psychologically stressful situation as the intervention concerns the male external genitalia, but open surgery seems to be even more stressful. To this effect, pharmacologic therapy generates less stress factors for patient and family environment. Malformations require sclerotherapy and/or resection[6] and in special cases pharmacological therapy.[20] Every treatment has advantages and disadvantages: As already discussed, the invasiveness of treatment plays an important role in the choice of procedure, especially in children. Sclerotherapy is favored due to its lower invasivity compared to surgery as surgery is a high-invasive procedure with a significant complication rate.[21] Further limitation of surgery is the dimension of the malformation and the needed expansion of resection.[22] Complete excision is often not possible due to technical or anatomical problems,[23] in this setting, sclerotherapy has technical superiority. In case of recurrence, surgery gets more complicated each time performed, and the rate of complications raises. Sclerotherapy may have a higher rate of punctures in repetitional interventions but seems to have no higher rate of procedural complications. Pharmacological therapy with mTOR inhibitors seems to be a promising option in the future, but at the moment it is restricted to studies and severe clinical cases.[20] In addition, a systemic pharmacological therapy has an expanded side effect profile (general immune system suppression, etc.) compared to local percutaneous sclerotherapy and a prolonged period of application (selective in sclerotherapy vs. long-term in pharmacological treatment). All these factors strengthen the importance of sclerotherapy.
Sclerosing agents recommended for VMs are mainly ethanol, (foamed) polidocanol, or sodium tetradecyl sulfate (STS); treatment of LMs is possible with OK-432, doxycycline or bleomycin.[18] The choice of sclerotherapy agent is influenced by different factors, particularly the anatomic region, the type and dimension of malformation and the flow criteria with a main focus on the venous outflow in VM.[24] Ethanol, polidocanol, ethanolamine oleate, and STS are the four mainly used agents in VM and are all effective as shown in two current systemic reviews.[14,24] Pure ethanol has the highest sclerosing power, polidocanol and STS are less aggressive and reduce morbidity,[24] ethanol gel has lower morbidity as well and improves the safety of ethanol[25] due to its enhanced local efficacy.[14] As genitalia is a delicate body region with fine tissue, too high sclerosing power can provoke complications. For this reason, pure ethanol was not the sclerosants of choice in this series. To treat LMs, a variety of sclerosing agents have been used, including ethanol, STS, OK-432, doxycycline, and bleomycin. OK-432 was found to be the first choice in a review article[26] with a high success rate,[27] but doxycycline and bleomycin seem to be safe and effective, too.[28,29] The macrocystic type and a complete aspiration of cystic contents play an important role for success.[30] We decided to apply OK-432 in this series because it is an effective first-line treatment with infrequent serious complications if mentioned that screening for an allergic reaction to penicilline is minded.[31]
Three of the patients included in this study had prior surgery. To no affect the sclerotherapy outcomes, the surgery had to be longer than 1 year in the past and was reported for the sake of completeness. Two of them had surgery independent of the vascular malformation (orchiopexy and hydrocele operation). One patient was operated in the past - independently of our study - on the suspicion of a dislocation of the testis, intraoperatively a LM was detected and lymph vesicles were excised, but LM was not eliminated and came back that demonstrates first the importance of correct diagnosis of a disease at best with MRI[32] and second the importance of correct choice of therapy. In the present case, sclerotherapy was the correct treatment because the underlying disease was CCLA.
As the most common vascular malformations are VM,[19] the reported vascular malformation cases of the male genitalia and their treatment options are almost exclusively VM. A Spanish study[33] describes the efficacy of treatment of VM of the glans penis with a laser in three patients. This case series reports a satisfying outcome, but a treatment indication was for esthetic reasons only. In our opinion, laser interventions should be used carefully in this delicate anatomic region due to a high risk of scarring, for example, at the glans penis that can result in urethral retraction.[34]
Another study on vascular malformations of the male external genitalia focused on sclerotherapy[35] of VM of the glans penis. 78% of these patients underwent sclerotherapy for cosmetic reasons, and only two patients were symptomatic (bleeding). The reported sclerosing agent was polidocanol 1–3% in liquid form. The applied volume varied from 2 ml to 4 ml per injection and 44% of patients needed multiple sclerotherapeutic sessions. No major complications were reported; however, cutaneous blistering occurred in 33% as a minor complication due to inflammatory reactions. Cutaneous blistering did not occur in this series. This may be due to the administration as sclerosing foam or a reduced local concentration compared to other studies.
In this study, 50% of patients suffered from LM, 50% simple LMs, and the residual 50% suffered from LMs associated with syndromes.
The reported pathology of localized genital LM and its treatment was typically idiopathic.[36] However, as our series shows this condition can be part of syndromic overgrowth disorders as well: 2 patients of our study group suffered from CLOVES syndrome. This syndrome with segmental overgrowth is associated with venous and lymphatic or combined vascular malformations.[37,38] One patient was diagnosed for CCLA, defined by the dysgenesis of central lymphatic channels of the body leading to lymph stasis and increased lymphatic pressure in dependent areas of the body including the genitalia. In this study, patients with lymphedema were especially examined with an intranodal lymphangiography to verify or exclude a problem in the central conducting lymphatic tract. Literature of percutaneous sclerotherapy on LMs is mostly focused on other body regions, for example, the cervicofacial area as the most common location.[39] Reports of LMs of the male genitalia are case reports[40,41] and very rarely focused on sclerotherapy.[23] In a Dutch study, three patients with lymphedema and lymphatic leakage at the penis and scrotum were referred to surgical treatment.[42] In two cases, a circumcision was necessary for a meticulous excision of anatomic structures. Regarding the third patient, the lymphedema was associated with recurring infections which subsided after surgical treatment, whereas leakage persisted.
One limitation of the current study is the small number of patients due to the combination of a rarely affected anatomic location with a seldom executed interventional treatment in this body area in the indication of a rare disease. Certainly, a multicenter study would be desirable to provide more data. The effectiveness of the different sclerosing agents used was not compared to each other in this study due to the small patient numbers in each group.
In summary, this study on sclerotherapy treatment of the male external genitalia in vascular malformation patients provides information about sclerosing therapy with different sclerosing agents in this sensitive region of the human body. All patients benefitted clinically without complications, but only one patient reported a complete resolution of symptoms after treatment.
On behalf of all authors, the corresponding author states that there are no conflicts of interest.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Percutaneous sclerotherapy of vascular malformations in children using sodium tetradecyl sulphate: The birmingham experience. J Plast Reconstr Aesthet Surg. 2012;65:1451-60.
- [CrossRef] [PubMed] [Google Scholar]
- The efficiency of sclerotherapy in the treatment of vascular malformations: A retrospective study of 63 patients. Plast Surg Int. 2016;2016:2809152.
- [CrossRef] [PubMed] [Google Scholar]
- Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69:412-422.
- [CrossRef] [Google Scholar]
- Vascular anomalies of the female external genitalia. J Pediatr Surg. 2006;41:993-9.
- [CrossRef] [PubMed] [Google Scholar]
- Vascular anomalies of the male genitalia. J Pediatr Surg. 2011;46:1214-21.
- [CrossRef] [PubMed] [Google Scholar]
- Picibanil (OK-432) in the treatment of head and neck lymphangiomas in children. Dent Res J (Isfahan). 2012;9:S192-6.
- [Google Scholar]
- Lymphatische Malformationen im Kindesalter unter besonderer Berücksichtigung von Prognose und Spätergebnissen: Humboldt-Universität zu Berlin. Berlin: Medizinische Fakultät Universitätsklinikum Charité; 2005.
- A case-based approach to common embolization agents used in vascular interventional radiology. AJR Am J Roentgenol. 2014;203:699-708.
- [CrossRef] [PubMed] [Google Scholar]
- Ethanol sclerotherapy of peripheral venous malformations. Eur J Radiol. 2004;52:283-7.
- [CrossRef] [PubMed] [Google Scholar]
- Venous vascular malformations in pediatric patients: Comparison of results of alcohol sclerotherapy with proposed MR imaging classification. Radiology. 2002;223:639-44.
- [CrossRef] [PubMed] [Google Scholar]
- Ethanol sclerotherapy of venous malformations: Evaluation of systemic ethanol contamination. J Vasc Interv Radiol. 2001;12:595-600.
- [CrossRef] [Google Scholar]
- Ethanolgel sclerotherapy of venous malformations improves health-related quality-of-life in adults and children results of a prospective study. Eur Radiol. 2017;27:2482-2488.
- [CrossRef] [PubMed] [Google Scholar]
- Ethanol-gel sclerotherapy of venous malformations: Effectiveness and safety. AJR Am J Roentgenol. 2017;209:1390-5.
- [CrossRef] [PubMed] [Google Scholar]
- Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-13.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of lymphangiomas with picibanil in the first year of life. Klin Padiatr. 2008;220:248-52.
- [CrossRef] [PubMed] [Google Scholar]
- Advanced management of congenital vascular malformations (CVM) Int Angiol. 2002;21:209-13.
- [Google Scholar]
- Endovascular treatment of slow-flow vascular malformations. Tech Vasc Interv Radiol. 2013;16:12-21.
- [CrossRef] [PubMed] [Google Scholar]
- Interventional management of vascular malformations. Tech Vasc Interv Radiol. 2011;14:22-31.
- [CrossRef] [PubMed] [Google Scholar]
- Sirolimus in the treatment of vascular anomalies. Eur J Pediatr Surg. 2017;27:86-90.
- [Google Scholar]
- Vascular malformations of the upper limb: A review of 270 patients. J Hand Surg Am. 1999;24:1019-35.
- [CrossRef] [PubMed] [Google Scholar]
- Retroperitoneal and genital lymphangioma therapeutic challenges in a developing country. Libyan J Med. 2009;4:44-5.
- [CrossRef] [PubMed] [Google Scholar]
- Huge scrotal, flank, and retroperitoneal lymphangioma successfully treated by OK-432 sclerotherapy. Urology. 2002;60:1112.
- [CrossRef] [Google Scholar]
- Intramuscular venous malformations of the upper and lower limbs: Indications and outcomes of sclerotherapy. Cardiovasc Intervent Radiol. 2018;41:1505-12.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of venous malformations: First experience with a new sclerosing agent a multicenter study. Eur J Radiol. 2011;80:e366-72.
- [CrossRef] [PubMed] [Google Scholar]
- Sclerotherapy for lymphatic malformations in children: a scoping review. J Pediatr Surg. 2011;46:912-922.
- [CrossRef] [PubMed] [Google Scholar]
- Patients with lymphatic malformations who receive the immunostimulant OK-432 experience excellent long-term outcomes. Acta Paediatr. 2015;104:1169-73.
- [CrossRef] [PubMed] [Google Scholar]
- Doxycycline sclerotherapy in children with lymphatic malformations: Outcomes, complications and clinical efficacy. Pediatr Radiol. 2012;42:1080-8.
- [CrossRef] [PubMed] [Google Scholar]
- Bleomycin A5 sclerotherapy for cervicofacial lymphatic malformations. J Vasc Surg. 2011;53:150-5.
- [CrossRef] [PubMed] [Google Scholar]
- OK-432 sclerotherapy of lymphatic malformation in the head and neck: factors related to outcome. Pediatr Radiol. 2014;44:857-862.
- [CrossRef] [PubMed] [Google Scholar]
- Treatment of lymphatic malformations with OK-432 (Picibanil): Review of the literature. J Craniofac Surg. 2009;20:1159-62.
- [CrossRef] [PubMed] [Google Scholar]
- MR imaging of soft-tissue vascular malformations: Diagnosis, classification, and therapy follow-up. Radiographics. 2011;31:1321-40.
- [CrossRef] [PubMed] [Google Scholar]
- Venous malformation of the glans penis: Efficacy of treatment with neodymium: yttruim-aluminum-garnet laser. Urology. 1999;53:779-83.
- [CrossRef] [Google Scholar]
- Laser treatment of glans penis hemangioma. Eur Urol. 1993;24:81-3.
- [CrossRef] [PubMed] [Google Scholar]
- Sclerotherapy for venous malformations of the glans penis. Urology. 2001;57:310-3.
- [CrossRef] [Google Scholar]
- Male genital lymphedema: Clinical features and management in 25 pediatric patients. J Pediatr Surg. 2014;49:1647-51.
- [CrossRef] [PubMed] [Google Scholar]
- Somatic mosaic activating mutations in PIK3CA cause CLOVES syndrome. Am J Hum Genet. 2012;90:1108-15.
- [CrossRef] [PubMed] [Google Scholar]
- PIK3CA-related overgrowth spectrum (PROS): Diagnostic and testing eligibility criteria, differential diagnosis, and evaluation. Am J Med Genet A. 2015;167A:287-95.
- [CrossRef] [PubMed] [Google Scholar]
- Percutaneous sclerotherapy for lymphatic malformations: A retrospective analysis of patient-evaluated improvement. J Vasc Interv Radiol. 2006;17:1639-48.
- [CrossRef] [PubMed] [Google Scholar]
- Lymphangioma circumscriptum of the male genitalia. Indian Dermatol Online J. 2016;7:68-9.
- [CrossRef] [PubMed] [Google Scholar]
- Lymphangioma circumscriptum in the scrotum: a case report. J Med Case Rep. 2012;6:233.
- [CrossRef] [PubMed] [Google Scholar]
- Congenital lymphoedema of the genitalia. Eur J Pediatr. 1998;157:943-6.
- [CrossRef] [PubMed] [Google Scholar]






